A ketamine-based antidepressant will soon be available from medical clinics run by Toronto-based cannabis producer Aleafia Health Inc. (TSX: AH).
The psychedelic-based medication is made by Johnson & Johnson (NYSE: JNJ) and is approved by the U.S. Food and Drug Administration and Health Canada for the prescription of treatment-resistant depressive disorder.
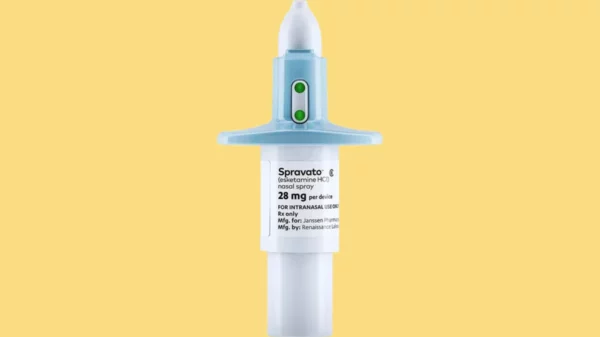
Spravato was first approved for use in Canada earlier this week and contains the drug esketamine, which is a type of ketamine. Both of these drugs are used in treatment-resistant depression therapies and can work to relieve symptoms faster than selective serotonin-reuptake inhibitors which are traditionally used to treat depression, according to the Psychiatric Times.
The drug esketamine, marketed as Spravato, has been shown to help people with major depression.
Now two studies suggest it can relieve depressive symptoms in people actively considering suicide. https://t.co/7kfFDHrZ11
— NPR (@NPR) September 10, 2019
The drug can currently be prescribed and administered by physicians and nurses at some Aleafia Health’s medical clinics as well as at Canabo Medical Inc. clinics, which are a wholly owned subsidiary of Janssen Pharmaceuticals, Inc., a division of Johnson & Johnson.
In a Friday statement, Aleafia Health said it is working towards being able to offer Spravato at its nationwide network of clinics and education centres, which to date have served over 70,000 patients.
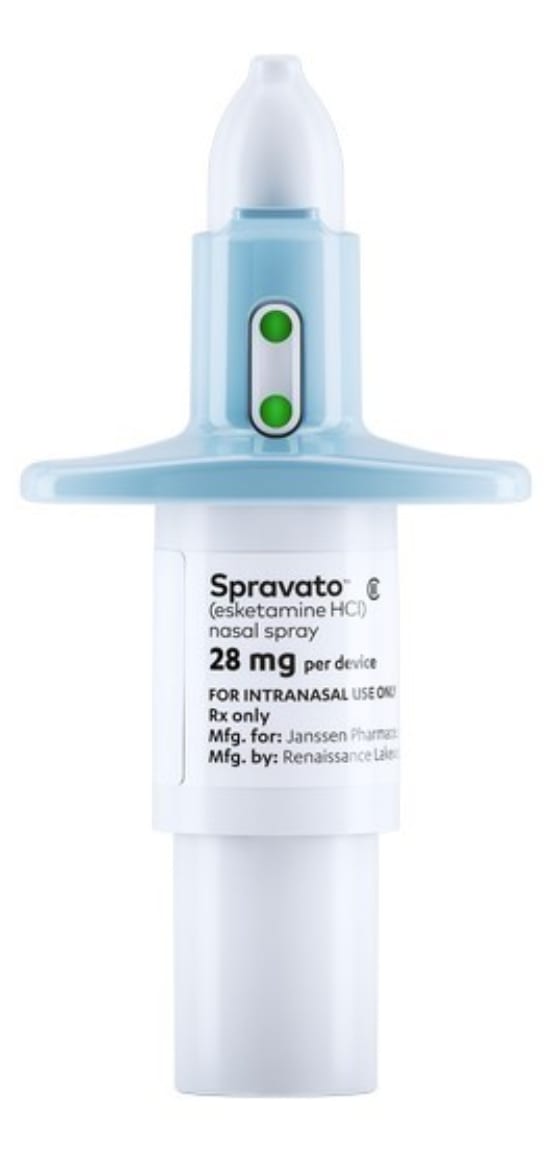
Spravato (esketamine) nasal spray. Press photo
The antidepressant is administered as a nasal spray. It was approved by Health Canada after it completed two Phase 3 clinical trials, a short-term induction study and long-term maintenance study, according to the statement.
For the study participants were shown to have statistically significant improvements in depression symptoms when they took Spravato and an oral antidepressant for four weeks, compared to participants who were given a placebo and an oral antidepressant for the same amount of time.
Aleafia Health CEO Geoffrey Benic said the company’s existing infrastructure of clinics and physicians made it particularly well situated to administer Spravato.
“The expansion of Aleafia Health’s services to our existing and prospective patients speaks to our commitment to improving the lives of Canadians through innovative, evidence-based medicine,” he said in a statement.
Psychedelics on their own are still illegal in Canada, but can legally be used by pharmaceutical companies as ingredients in medications, cannabis and psychedelic venture capitalist Henri Sant-Cassia told Mugglehead in May.
As pharmaceutical companies are already able to access psychedelics in this way, as well as many other ingredients that can’t be legally bought and sold in their raw form, Sant-Cassia says he doesn’t think the government will ever legalize psychedelics.
Read more: Psychedelics are experiencing a renaissance, but don’t expect them to be legalized anytime soon
Aleafia Health’s stock rose 14 per cent on the news to $0.65 per common share.
michelle@mugglehead.com
@missmishelle