Canopy Growth Corp. (TSX: WEED) has completed the first study to look at toxicity and life-long effects of chronic cannabidiol use. The results are promising — at least in worms.
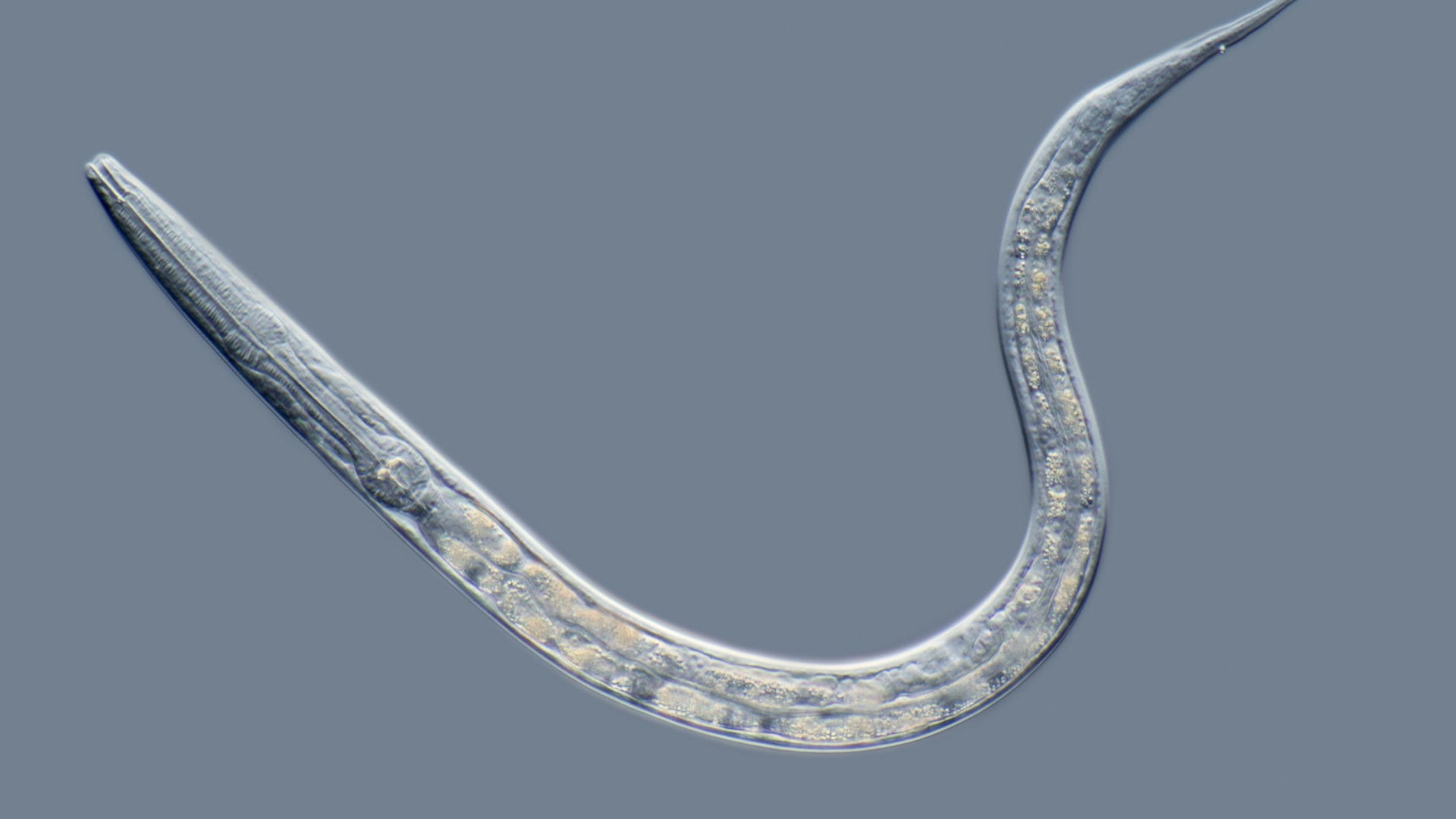
On Monday, the Canadian mega-producer reported that none of the 3,504 C. Elegans, also known as roundworms, that were given CBD showed any signs of toxicity or died prematurely compared to a control group, even at significantly elevated doses. Instead, CBD extended lifespan up to 18 per cent and increased late-stage life activity up to 206 per cent.
The C. Elegans are often used in early-stage research by the pharmaceutical industry to test novel compounds because 60-80 per cent of their genes are shared with humans and their short lifespan of two-to-three weeks makes the research more efficient.
But the study underscores a need for more CBD research and its life-long use on mammalian models, especially considering millions of people have been using it in the last two years despite a lack of long-term findings, says Hunter Land, a senior science director at Canopy.
Read more: Global CBD report underscores need for low-dose clinical trials
“These results serve as the only CBD life-long exposure data in an in-vivo model to date, and the absence of long-term toxicity gives us the evidence we need as an industry to continue researching the potential health benefits for the broader application of CBD,” he said in a statement.

Companies like Canopy Growth and Charlotte’s Web are funding CBD research to determine its safety and speed up US FDA regulations for the substance as a natural health supplement. Deposit Photos
CBD regulations and science remain in question
Canopy, which sells Martha Stewart-branded CBD gummies and tinctures in the U.S., is one of many cannabis companies trying to fill the research gap on the compound’s long-term safety to help guide the country’s Food and Drug Administration on how to regulate it.
Last week, the federal agency said the substance has shown varying effects in women and men, which adds more unanswered questions and will influence its regulatory timeline.
The industry has been awaiting federal rules for CBD since hemp was legalized in the U.S. at the end of 2018. Unlike its cannabinoid cousin THC, CBD is considered non-intoxicating and is widely used to treat conditions like anxiety and insomnia.
However, the FDA banned marketing the health benefits of CBD in 2019, and warned the public in March about potential harms including liver injury, as well as interactions with other drugs.
Charlotte’s Web (TSX: CWEB), the leading hemp-based CBD brand in the U.S., sponsored the first study in June to explore the effects of daily use on the liver in healthy adults. Along with six other CBD brands, the goal is to provide third-party scientific data from the completed study directly to the FDA by the end of this year.
Read more: Charlotte’s Web backs study on health outcomes of CBD
While CBD regulations remain murky in the U.S., the industry got a big boost across the Atlantic last week when the European Union’s top court ruled that hemp-derived CBD is not a narcotic drug.
“The CBD at issue in the main proceedings does not appear to have any psychotropic effect or any harmful effect on human health on the basis of available scientific data,” the ruling reads.
Canopy’s pre-clinical study was published in the journal Cannabis and Cannabinoid Research, and was conducted through its medical division Spectrum Therapeutics in partnership with NemaLife Inc., a life-extension research firm based in Texas.
Top image via Artis Micropia
jared@mugglehead.com
@JaredGnam














Kushly
November 27, 2020 at 7:23 am
Hello! Thank you for covering such important topics in the field of CBD, and it was a discovery for me that CBD affects men and women differently, it would be interesting to know how the effect is different. If it’s really true that CBD prolongs a person’s lifespan by 18 percent, I think a lot of people will immediately rush to buy CBD products. This is truly a great discovery. I hope this effect of CBD will not only be on worms but on humans too.