Cancer is everywhere.
It stalks us on the subway in ads for drugs, and it’s played an indelible part of our culture, and for many of us, it’s also in our families.
The disease ranks as the second leading cause of death globally, just behind cardiovascular diseases. According to the World Health Organization (WHO), the disease was responsible for nearly 10 million deaths worldwide in 2020. That’s one in six deaths.
The rise in cases is driven by various factors, including aging populations, increased exposure to carcinogens, and lifestyle choices. Lung cancer, colorectal cancer, and liver cancer remain among the most deadly forms. Despite advancements in early detection and treatment, many people still face challenges in accessing timely care. This also contributes to the high mortality rates.
In 2024, the American Cancer Society (ACS) projects that one in four men and one in five women in the U.S. will develop the disease at some point in their lives. This translates to an estimated 1.1 million new cases in men and about 920,000 new cases in women for the year.
Lung cancer is the most frequently diagnosed in men, followed by prostate and colorectal. In women, breast cancer leads in diagnosis, followed by lung and colorectal cancers.
In terms of mortality, the statistics reveal a similarly stark reality. Approximately 611,000 deaths from the disease are expected in the U.S. this year. For men, the death rates are highest for lung cancer, which accounts for about one in five cancer deaths. In women, breast cancer remains the leading cause of cancer death.
Read more: Breath Diagnostics takes aim at lung cancer with One Breath
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Global cancer-fighting market continues to grow rapidly
According to the ACS, there were around 20 million new cases worldwide in 2022, resulting in approximately 9.7 million deaths. Fortunately, the cancer-fighting market is also substantial, driven by the increasing need for innovative treatments and preventive measures. As such, prevention strategies and improved access to early detection and treatment have grown in importance.
How big is this market?
The global market for treatments and technologies for the disease continues to grow rapidly. It’s driven primarily by innovations in immunotherapy, targeted treatments, and early diagnostics.
In 2023, the global oncology market was valued at around USD$200 billion and is expected to expand significantly in the coming years. This growth reflects not only the increasing prevalence of cancer but also advancements in medical technologies, drug development, and personalized treatment approaches that offer new hope for patients. Investments in research and treatments remain crucial as the world seeks to curb the growing burden of the disease.
And there are plenty of companies out there working to find a way to bring those dire numbers under control.
For example, urine tests offer a promising, noninvasive way to detect it, according to Philip Abbosh, MD, PhD, of Fox Chase Cancer Center. At the AACR Special Conference.
Abbosh emphasizes the potential of the tests to revolutionize patient care. His team is developing a test to detect residual bladder cancer after neoadjuvant therapy, aiming to help some patients avoid bladder removal.
Neoadjuvant therapy refers to treatments given before the main treatment, often surgery, to shrink a tumor or reduce its spread. It typically involves chemotherapy, radiation therapy, or hormone therapy, depending on the type.
Read more: Tilray to provide cannabis for Spanish clinical study on severe brain cancer
Read more: Filament Health gets FDA approval for psilocybin studies on cancer-related anxiety and depression
ARPA-H aiming for at-home cancer detection tests
Despite the promise, Abbosh acknowledges the challenge of ensuring these tests achieve high accuracy before they can be widely used in clinical settings.
Although only a few urine-based cancer detection tests are FDA-approved, research continues to expand. A study in the International Journal of Cancer found over 900 articles between 2010 and 2022 mentioning urine biomarkers for various cancers.
Advanced Research Projects Agency for Health (ARPA-H), through its POSEIDON program. The program aims to develop at-home tests to detect over 30 cancers in their early stages using urine or breath samples. This initiative aligns with the Biden Cancer Moonshot’s goal to “end cancer as we know it.”
Furthermore, researchers are also exploring new urine-based methods for detecting lung cancer in never-smokers. At present, current screening methods focus primarily on smokers. Studies have identified biomarkers like creatine riboside (CR) and N-acetylneuraminic acid (NANA), which show elevated levels in both smokers and nonsmokers with lung cancer.
Another study tested an inhalable nanosensor system that could identify lung cancer biomarkers in urine. So far, this test has shown promise in mouse models.
Additionally, in ovarian cancer research, a team developed a “gold fingerprint” approach using nanopore sensing to detect peptides in urine that indicate cancer. This offers a simpler and more cost-effective detection method.
Meanwhile, a team studying pancreatic cancer developed a urine biomarker panel that showed high sensitivity and specificity in detecting cancer up to two years before diagnosis.
Bladder cancer detection could benefit from combining blood and urine tests. Lars Dyrskjøt, PhD, from Aarhus University Hospital, said that circulating tumour DNA (ctDNA) and urine tumour DNA (utDNA) work best together in predicting treatment response and survival. The study found that a negative result in both blood and urine tests correlated with an excellent patient outcome.

Poseidon. Image via ARPA-H.
American Cancer Society recommends lung cancer screenings
Non-profit, 14-hospital academic medical system Allegheny Health Network (AHN) and DELFI Diagnostics, Inc. have partnered to assess the effectiveness of DELFI Diagnostics’ ‘FirstLook’ blood test in enhancing lung cancer screening for at-risk individuals in western Pennsylvania.
The American Cancer Society recommends annual lung cancer screenings with low-dose CT scans for people aged 50 to 80 with a 20 pack-year smoking history. However, many eligible patients do not undergo screening, often due to a lack of awareness or reluctance to get a CT scan.
“Whether it’s due to lack of awareness that screening is available or reluctance to undergo CT scanning, there are millions of Americans who should be getting screened for lung cancer each year who aren’t,” said Ali H. Zaidi, MD, medical director of aerodigestive research at AHN Cancer Institute.
“The FirstLook blood test may help us better identify those who are most at risk of this disease and who would benefit from follow-up CT screening.”
For the next 24 months, AHN researchers will evaluate if the FirstLook blood test improves lung cancer screening adherence. The test will be available through AHN’s pulmonary and primary care practices and, later, at community-based cancer screening events.
The FirstLook test uses whole-genome machine learning to analyze cell-free DNA fragments in the blood that are markers for cancer. Previous trials demonstrated the test’s 99.8 per cent negative predictive value. This means it’s highly unlikely a negative result will later show lung cancer on a CT scan.
Read more: Alpha Tau treats first lung cancer patient with radiation therapy in Jerusalem
Read more: American VA reminds Veterans to get screened for lung cancer
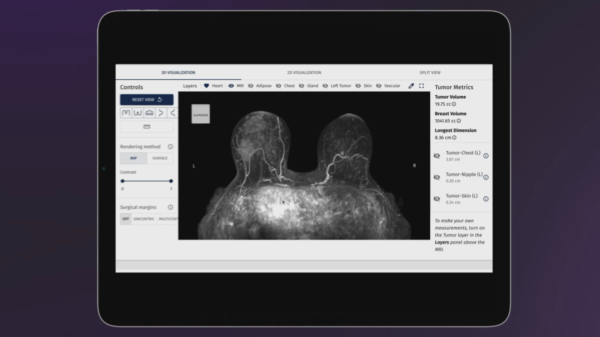
Breath Diagnostics analyzes volatile organic compounds in human breath
Outside of the non-profit sector, there are companies putting money into doing serious research to take aim at the disease.
Breath Diagnostics, Inc., for example, specializes in developing non-invasive breath analysis technologies for early disease detection. By analyzing volatile organic compounds (VOCs) exhaled in human breath, the company aims to identify biomarkers that can indicate the presence of cancer at an earlier stage than traditional diagnostic methods.
Their innovative technology offers a safer and more accessible alternative to conventional screenings like biopsies and CT scans. Further, Breath Diagnostics’ tests work by collecting a breath sample from the patient and analyzing the VOCs using advanced sensor technology and algorithms to detect abnormalities.
Additionally, Breath Diagnostics is exploring the potential of its breath analysis technology to detect other diseases, including gastrointestinal and metabolic conditions. This versatility could make breath-based diagnostics a powerful tool across multiple areas of healthcare.
Breath Diagnostics aims to enhance early diagnosis and treatment by making disease detection easier. Their technology has the potential to revolutionize how diseases are diagnosed and managed in clinical settings.

Breath Diagnostics ‘one breath’ technology.
New medical tech helps boost economic growth in UK life sciences
The UK is set to trial a wave of new UK-created cancer therapies. Public and private sector investments will back partnerships aimed at faster diagnoses and better treatments for cancer and other diseases, using cutting-edge technologies.
This initiative could lead to the global release of flexible medical scanners and an AI tool for earlier detection.
These medical technologies promise extend patients’ lives and also boost economic growth through the UK’s leading life sciences sector.
Their integration is essential for modernizing the NHS, a priority highlighted by the Darzi Review, which exposed England’s lagging survival rates from the disease. Additionally, the government’s investment package will be central to addressing these issues, focusing on adopting innovative solutions and transforming how the NHS delivers care to patients.
“Cancer is a disease that has brought pain, misery and heartbreak to every family in the country, including my own,” said Peter Kyle, Science and Technology Secretary.
“But through government working in partnership with the NHS, researchers, and business, we can harness science and innovation to bring the detection and treatment of this horrendous disease firmly in to the 21st century, keeping more families together for longer.”
UK Research and Innovation has announced a £118 million fund to create five new hubs across the country aimed at developing new health technologies.
The funding will come from both government and partner support.
.
Follow Joseph Morton on Twitter
joseph@mugglehead.com