Everyone knows someone who has been touched by cancer.
Whether it be directly through a diagnosis, or through the impotent agony of watching a loved-one deal with the disease.
According to the World Health Organization (WHO), cancer has become a leading cause of death in many non-Western countries. In nations within parts of Asia, Africa, and Latin America, cancer has also surpassed infectious diseases as the top cause of mortality.
Among the more pernicious types of cancer—these being colorectal, breast, prostate, lung and pancreatic—lung cancer receives the least attention.
Furthermore, according to the American Lung Association, 20 million Americans are eligible for lung cancer screenings and almost none get them. There are 94 million current and former smokers in the United States, with many of these at high risk.
Early detection is crucial in improving survival rates and the chances of successful treatment for lung cancer. Unfortunately, the 5-year survival rate for lung cancer is only 18.6 per cent. This is significantly lower than other leading cancers like colorectal (64.5 per cent), breast (89.6 per cent), and prostate (98.2 per cent).
When the disease is detected while still localized in the lungs, the survival rate improves to 56 per cent. Unfortunately, only 16 per cent of lung cancer cases are diagnosed at this early stage.
However, there is a new technology presently in development that could put a dent in those numbers.
Read more: Tilray to provide cannabis for Spanish clinical study on severe brain cancer
Read more: Filament Health gets FDA approval for psilocybin studies on cancer-related anxiety and depression
One Breath is easy to use
The technology is called One Breath and it’s being developed by a company called Breath Diagnostics Inc.
It’s easy to use.
A patient breathes one breath into a bag. The bag captures the volatile organic compounds (VOC) present in a breath, which include biomarkers associated with lung cancer. This is bag is then attached to a machine that sucks the air from the bag and concentrates the VOC’s onto a microreactor chip. This chip stabilizes the VOCS and the samples can then be examined in a laboratory.
The most commonly used breath collection technology today is something called thermal desorption tubes.
The difference between thermal desorption tubes versus Breath Diagnostics’ microreactor chip is that the chip stabilizes and captures the VOCs through a chemical process. Unlike thermal desorption tubes, which require a desorption process to release the samples for analysis, OneBreath’s chemical process forms molecular compound compositions called adducts. In chemistry, these adducts are cationic, which means they carry a positive electrical charge. This positive charge helps a machine called a mass spectrometer with detection and analyses.
Analyzing these adducts with a mass spectrometer significantly enhances sensitivity. This advancement allows them to measure and quantify trace levels of substances, rather than merely detecting VOCs.
After the trapped adducts have been released, the company can employ cold solvent rinsing to the trapped VOC’s and see what there is to see. It doesn’t require “heating up” of the sample, which could lead to thermal degradation. In other words, Breath Diagnostics’ accuracy of quantifying VOCs – and not just identifying them – separates it from the most commonly used breath analysis technologies today.
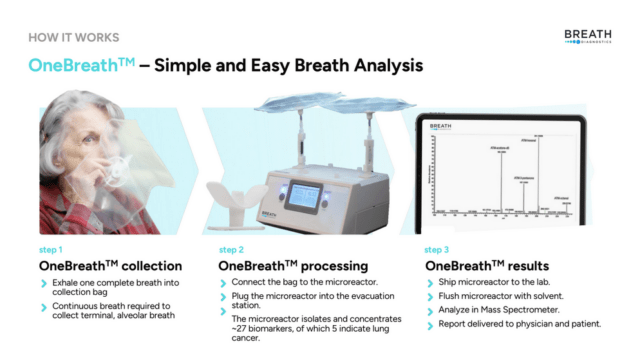
Image via Breath Diagnostics.
Current care standard not without risk
Lung cancer testing compliance has also proven tricky.
The problem is that the methods presently being used are invasive, costly and dangerous, according to Breath Diagnostics CEO, Ivan Lo.
“The compliance rate for lung cancer is 6 per cent versus 65 to 85 per cent for all other major cancers. Yet lung cancer kills more people than the top four cancers, both in Canada and the US, combined,” Lo said.
The problem is that the present standard of care involves something called a low-dose CT (LDCT) scan.
An LDCT is a medical imaging technique that uses ionizing radiation to create detailed images of the body. Unlike standard CT scans, which deliver higher doses of radiation, LDCT scans reduce the amount, making them useful for screening.
This method is commonly employed for early disease detection in high-risk populations. Using lower doses of radiation, LDCT scans balance the need for clear, diagnostic images with the goal of reducing potential harm from radiation exposure. The problem is that LDCT still delivers 20 times more radiation than an X-ray.
Furthermore, lack of awareness, limited access to screening, overdiagnosis, and cost all contribute to the challenges of lung cancer detection.
It’s also far from foolproof. LDCT may miss some small lung cancers or nodules, leading to false-negative results, while false-positive results are extremely common.
For example, 40 per cent of at-risk individuals show concerning results but only 2 per cent of those screened positive actually have lung cancer. Diagnostic follow-ups can run patients between USD$3000 and USD$15,000 each, often requiring multiple LDCT scan follow-ups.
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Read more: Artificial intelligence proven to help in cancer screening
Low-dose CT scans may increase lung cancer mortality
The National Lung Screening Trial (NLST), a large randomized controlled trial performed in 2014 by the National Cancer Institute, revealed a higher rate of false-positive results among those who underwent LDCT screening compared to those who received a chest X-ray.
This trial also reported increased rates of overdiagnosis and overtreatment associated with LDCT screening for lung cancer.
Three years later, a study published in the Journal of the American Medical Association (JAMA) found that LDCT screening for lung cancer was linked to a small but statistically significant increase in lung cancer mortality due to radiation exposure.
The study determined that the risk of lung cancer mortality due to radiation exposure was 1.6 deaths per 10,000 person-years in the LDCT screening group. This was compared to 1.3 deaths per 10,000 person-years in the chest X-ray screening group.
Lo continued in saying that the company has already test its technology through multiple studies involving over 800 patients and across three clinical sites. However, he does admit that Breath Diagnostics isn’t ready to start the approval process to get authorization from the Food and Drug Administration (FDA) quite yet.
“Our goal right now is to commercialize the product before FDA approval, through a laboratory developed test,” Lo said.
New microreactor may improve accuracy
The logic behind commercialization involves selling the product in the private markets.
“All you have to do is confirm that your results are what you say they are, get CLIA certified, and people can choose to pay for it. We want to prove that any third party lab with access to mass spectrometers, such as the Mayo Clinic, can repeat our results,” Lo said.
The idea is to use that framework to conduct a National Lung Cancer Screening Trial. Lo insists this will take two to three years due to the current standard of care and the low disease prevalence rate for lung cancer. The current standard of care for screening is LDCT often requires multiple follow-ups over a period of time to determine if a patient truly has lung cancer.
Lo’s goal right now is to commercialize the technology and begin the process of a national lung cancer screening trial within the 12-18 months.
Breath Diagnostics is also launching a new version of the microreactor chip that should improve sensitivity and accuracy.
Read more: Microsoft and AI company join forces to build cancer diagnosis platform
Read more: WELL Health and Healwell AI join forces to produce more efficient hospitals
Anyone touched by cancer may invest
The company anticipates that returns on this technology could be tremendous.
“We have the potential to go through the FDA process for less than USD$20 million, while FDA trials for therapeutics can cost upwards of a billion dollars. Yet, the potential ROI of a new lung cancer screening technology can be in the billions – rivalling therapeutics and drugs.” Lo said.
That’s one of the reasons the company is circumventing the traditional cash raising models like accepting money from micromanaging venture capitalists, and instead filing something called a Form C with the Securities and Exchange Commission (SEC).
A Form C is a regulatory document that companies use to conduct a securities offering under regulation crowdfunding.
Typically, investment is limited by regulation to accredited investors. The Form C gets around that by letting anyone touched by this disease get in on the action.
Lo believes it will be successful and that investors will be rewarded by being able to invest in a company that can change the landscape of lung cancer diagnostics.
For now, though, the push is to get screened early, even if there are no symptoms.
“We believe, with this technology, even before FDA approval, that we can convince people to just breathe in a bag for 30 seconds. I think they’ll do it,” Lo said.
It only takes one breath to potentially save a life.
.
Follow Joseph Morton on Twitter
joseph@mugglehead.com














