Insomnia sufferers could soon have a new treatment option without the undesirable side effects of other available drugs.
On Wednesday, cannabis medicines developer Zelira Therapeutics Ltd. (ASX: ZLD and OTCQB: ZLDAF) announced the positive results of a recent clinic trail at the University of Western Australia.
Zelira halted the trading of its stock on the Australian Stock Exchange on Monday pending the trail results. Trading has now resumed with little change to its valuation at AU$.054 per share.
The study of the ZTL-101 treatment concluded that the cannabis-derived drug was safe, well tolerated and significantly reduced insomnia.
Professor Peter Eastwood, director at the Centre for Sleep Science, expressed his enthusiasm over the results in a press release issued by the company.
“This study represents the most rigorous clinical trial ever undertaken to assess the therapeutic potential of medicinal cannabis to treat the symptoms of chronic insomnia,” he said.
The professor, who was also the study’s principal investigator, also noted it was the first trial to use the Insomnia Severity Index (ISI) — arguably the gold standard in the field — to measure the effectiveness of a medical cannabis product to treat insomnia.
“The fact that ZLT-101 treatment achieved a statistically significant improvement in ISI scores is very impressive, particularly given the relatively short two-week dosing window,” he said. “The lack of serious adverse or persistent mild adverse events is also encouraging given the reported safety issues for several already approved insomnia therapies.”
Taken together, he said the results suggest ZTL-101 has the potential to be a novel insomnia treatment.
Read more: Health Canada approves two new Tetra Bio Pharma topicals for over-the-counter sales
The trail recruited 24 chronic insomnia patients aged 25-70 taking the medication and a placebo for 14 nights each on a randomized basis. Participants could choose a single 11.5 milligram cannabinoid dose or a double dosage. Over half the participants chose to double down.
A total of 36 “non-serious adverse events possibly or likely related to ZTL-101” were recorded from 17 participants. Most frequently reported were dry mouth (22.2 per cent) dizziness (16.7 per cent), headache (11.1 per cent) and feeling abnormal (11.1 per cent).
All adverse events were classified as mild and had either resolved overnight (97.5 per cent) or soon after waking or were resolving at the end of the trial, according to the results.
Importantly, the treatment also showed a significant reduction in insomnia symptoms.
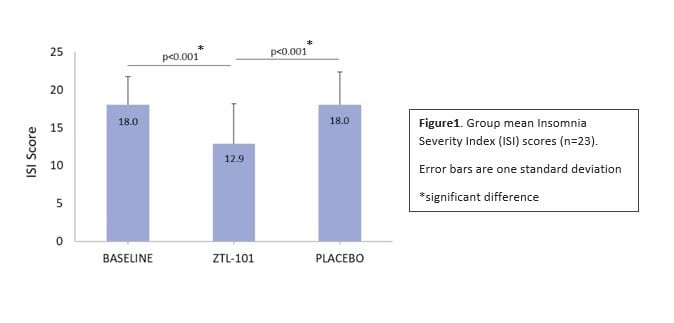
Trail results show a significant reduction (12.9±5.3, p<0.001) in participants’ Insomnia Severity Index scores. Figure courtesy of Zelira
Zelira chairman Osagie Imasogie said the positive outcome to this trial represents an important milestone for his company and its commitment to address the unmet need for clinically validated cannabis medicines and offer more treatment options to physicians and patients.
An estimated 70 million Americans have insomnia and the market for prescription and over-the-counter medications used to treat the condition generates over US$2 billion in annual revenue, according to the release.
A final report from the clinical study including an analysis of a comprehensive suite of secondary endpoints will be provided by end of March 2020, the company stated. These results will inform the design of any future clinical studies.
The company also has trials underway for chronic pain and autism treatments, according to its website.
Top image via Deposit Photos
nick@mugglehead.com
@nick_laba














