ChitogenX (CSE:CHGX) (OTCQB:CHNXF) finished its Phase I/II rotator cuff tear repair clinical trial.
The U.S. Phase I/II clinical trail is a blinded, randomized controlled study looking into the safety of ORTHO-R for rotator cuff tear repair, compared with standard of care for 78 patients at ten clinical sites throughout the United States safety phase.
“Successful completion of the initial safety portion of our U.S. clinical trial is a key milestone for our clinical development program and for the Company. With 9 of the 10 sites now actively recruiting, we look forward to accelerating enrolment of the required remaining patients and reporting the findings of our Phase I/II clinical trial to the FDA”, said Philippe Deschamps, CEO of ChitogenX.
ChitogenX is a clinical stage regenerative medicine company developing therapeutic tissue repair technologies. Specifically, its product is the RESTORE technology platform, a muco-adhesive Chitosan based biopolymer matrix that delivers biologics like platelet rich plasma (PRP) or bone marrow aspirate concentrate to assist healing in various regenerate medicine applications.
Read more: The Mugglehead technology roundup: medical tech and biotechnology edition
Read more: Delix gets recognized as leading start-up by Nature Biotechnology
The rotator cuff and clinical trial
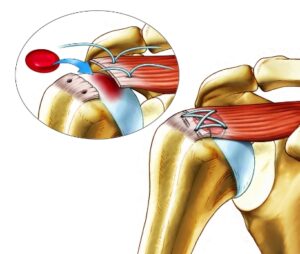
Rotator cuff/Image via ChitogenX
The rotator cuff refers to the four tendons that stabilize the shoulder joint. The joint is a ball and socket joint with a shallow socket requiring extra stabilization by the tendons of the four major muscles present. These tendons are frequently torn through repetitive stress over time. This also includes overhead activity like heavy lifting can cause these tears. Common symptoms include dull, aching pain, loss of sleep and weakness in the arms from lack of exercise.
The usual treatment involves surgery but these aren’t always successful. ChitogenX designed ORTHO-R to work with a patient’s blood to enhance the biological repair of the torn tendon.
“Every year, hundreds of thousands of patients undergo rotator tear repair surgery with a significant percentage still experiencing a high failure rate. We believe ORTHO-R to be the ideal biopolymer transport system and scaffold to provide the needed residency for biologics delivered to repair sites to help address this and other high unmet medical need,” said Deschamps.
The trial required staggered enrolment of five patients with a data safety monitoring committee sign-off for each trial participant. The recruitment phase of each trial involved a week waiting period after each case. This gave the committee time to determine required changes or stop the trial because of unforeseen safety issues. No issues arose and all approved U.S. clinical sites can continue.














