It’s October and for the past few years that’s meant masks and wait on your vaccine shot. It’s also meant hesitancy, political division and limitation on what you can and cannot do with your day. This October seems to be different. There’s been no mask mandate, nor have there been any closures, and that’s mostly to do with the gradual weakening of the variants of the COVID-19 virus. But that doesn’t mean we’re through the woods and out the other side. The variant strains may be weaker, but there are still dangers attached. It’s good for us that there are medical tech and biotechnology companies out there in the space looking for cures and treatments better than those presently on the market.
That’s also not to disregard the host of other health related issues we face as a society every winter.
Here are five biotechnology and medical tech companies pooling their resources to make our lives easier.
Medical tech and the next stage of COVID-19 vaccinations
Novavax (NASDAQ:NVAX) presented data from its phase 3 PREVENT-19 trial and study 307 at the World Vaccine congress Europe 2022.
The data from adults and adolescents aged 12 to 17 showed the company’s prototype COVID-19 vaccine (NVX-COV2373) met its immunologic endpoint. Also, study 307 met its objective, showing that three lots of the vaccine tested as a successful booster for immune responses in previously vaccinated adults.
“These data further demonstrate the consistent immunogenicity and safety profile of the Novavax COVID-19 vaccine as a booster, regardless of previous vaccine history. These data are an early indication that our vaccine may be effective against variants such as Omicron. We have ongoing trials further exploring the Novavax COVID-19 vaccine’s potential as an effective booster against these variants, including BA.4/5, and look forward to sharing these data,” said Gregory M. Glenn, M.D., president of research and development for Novavax.
Novavax wants the world to be a healthier place and its working towards that end. It does this through the discovery, development and commercialization of vaccines for serious infectious diseases. The company uses a recombinant technology platform to produce drugs that boost the immune system in response to global health needs.
The Novavax COVID-19 vaccine is a protein-based vaccine engineered from the genes of the first SARS-CoV-2 strain. The company put the vaccine together using its recombinant nanoparticle technology to generate antigen from the original coronavirus spike protein. The company reformulates the antigen to enhance the immune response and help with developing high levels of neutralizing antibodies.
Multiple regulatory bodies across the world, including the FDA, the WHO and the European Commission, have given the sign off on the company’s COVID-19 vaccine. It’s presently under review by more.
Also, Novavax is working on a COVID-19-Influenza combination vaccine. It’s presently in a Phase 1/2 clinical trial.
The PREVENT-19 trial involved an adult receiving a booster dose approximately eight or 11 months after the primary dose. The anti-spike immunoglobulin G, which is a type of antibody, levels increased relative to pre-boost levels.
They rose significantly, with neutralizing antibodies increasing by 34 to 27 fold compared to pre-boost levels when boosted at eight or 11 months.
The booster evaluations were the same in the version of the PREVENT-19 expansion given to adolescents between 12 and 17. The tests showed a significant antibody boost against Omicron BA.1, BA.2, and BA.5.
The Vitamin Shoppe offers free snack for a vaccine
The Vitamin Shoppe, a subsidiary of Franchise Group (NASDAQ:FRG), is offering a free protein bar or healthy snack to anyone who can prove they have gotten their flu vaccine.
The name of the campaign is “Snax for Vax” and it supports healthy communities during the flu season. It isn’t quite as catchy as vax and scratch or shot and a beer, which were a free scratch lotto card or a free beer for getting the COVID-19 vaccine, but if it gets people out, then it’s probably doing its job.
“The Vitamin Shoppe is dedicated to the lifelong wellness of our customers and the communities we serve. Public health experts have advised that the current flu season may be severe, so we want to encourage Americans to engage in healthy habits, such as getting an annual flu vaccine and supporting their immune systems with a healthy diet, regular exercise, and proper sleep routines. Our Health Enthusiast store teams look forward to our Snax for Vax weekend and giving away some of our most popular, protein-packed snacks as a tasty and healthy reward for supporting your wellness with a flu vaccine,” said Sharon Leite, CEO of The Vitamin Shoppe.
The Vitamin Shoppe is a omnichannel specialty retailer and wellness lifestyle company. It’s based in Secaucus, New Jersey, and offers an assortment of nutritional offerings, including vitamins and minerals, supplements and herbs, sports nutrition and homeopathy remedies. It also carries products from approximately 700 national brands. It also has its own brands, including The Vitamin Shoppe, Vthrive The Vitamin Shoppe, BodyTech, BodyTech Elite, fitfactor, fitfactor KETO, plnt, ProBioCare, True Athlete, and TrueYou.
The campaign kicks off on October 22-23 wherein anyone who gets a recent flu vaccine complete with proof from a doctor, pharmacist or other healthcare provider, can pick a protein bar or snack from any of the 700 The Vitamin Shoppe and Super Supplements stores in the U.S. and Puerto Rico. No purchase required.
Some of the options include:
- AP Regimen – PrimeBites Protein Brownies
- FULFIL – Vitamin & Protein Bars
- Garden of Life – Organic Fit High Protein Bars and Organic Performance Protein Bars
- Jacked Factory – Authentic Protein Bars
- Legendary Foods – Protein Sweet Rolls and Tasty Pastries
- ON – Protein Wafers, Vanilla Crème Flavor
Read more: The Mugglehead technology roundup: alternative medicine options edition
Read more: Transforming System licenses platforms for greater operational efficiency: NHS
Scotiabank collects a reward for their commitment to mental health
Excellence Canada awarded Scotiabank (TSX:BNS) (NYSE:BNS) with the Gold Standard Award for its role in creating a psychologically safe environment.
Depression causes an estimated 200 million lost workdays, and costs $17 billion to $44 billion to employers, according to the Center for Disease Control (CDC). Half of employees suffering from depression go untreated. The disease effects the absentee rate, causes loss of productivity, and effects the overall climate in the workplace. That’s why this is important.
“World Mental Health Day provides an opportunity for us to listen and reflect on the importance of the mental health and well being for all of our employees. The recognition from Excellence Canada is a proud moment for us as an employer, recognizing the culture we strive to create for employees,” said Barb Mason, group head and chief human resources officer for Scotiabank. “By supporting and empowering employees to take care of their mental health and wellbeing, and speak up about how they’re feeling, we’re creating a workplace where everyone can thrive.”
Scotiabank has made a number of different contributions to maintaining sustained mental health, including:
- Increasing bank paid coverage under the Canadian Benefits Plan from $3,000 to $10,000 for employees and their dependants this year.
- Enhanced caregiving support in terms of elder and childcare, including five childcare days per child (and elder) annually.
- Increasing availability for psychological services, including 24/7 helplines and individual consultations, at no additional cost to employees.
- Establishing a new global minimum standard for parental leave in early 2022. Including eight fully paid weeks for all parents with a new child, and eight more fully paid weeks for parents who have given birth. The standard will be completely implemented in 2025.
- Improved and increased access to mental health courses, webinars and workshops. Employees will get access to educational opportunities and how to improve their mental health and well-being. These include items like emotional intelligence and resilience, stress management and dealing with the new normal. These opportunities come with access to digital resources like internal guides, newsletters, posters and videos.
BioLife Solutions finds a cold chain solution for cell and gene therapy
Bioproduction tool and service provider for cell and gene therapy (CGT), BioLife solutions (NASDAQ:BLFS), and temperature control shipping solution provider Csafe inked a partnership to provide a global service net in support of CGT products.
The overall goal of the partnership is to increase reliability, enhance security and improve quality. Csafe is joining BioLife’s global network of cold chain solution providers in the CGT market. The numbers bear out to 10,000-12,000 shipments of CGT materials and manufactured doses over a year. Specifically, the new partnership provides expanded supply chain options for any CGT product at any stage of development.
“CSafe is honored to partner with the team at BioLife Solutions, whose range of CGT bioproduction tools and services is peerless in the market. We are excited to merge these exceptional tools and services with our expertise in global, reliable scaled delivery. CGT is hitting its stride and needs global support. We know how important these therapies are to patients everywhere, and it’s our mission at CSafe to protect every shipment,” said Patrick Schafer, CSafe CEO.
BioLife Solutions is a supplier of bioproduction tools and services for CGT and other biopharma markets. Its tools include the CryoStor and HypoThermosol biopresevation media for shipping and storage. Additionally, the ThawSTAR family of automated, water-free thawing products, and the evo cold chain management system.
In contrast, Csafe offers thermal shipping solutions for pharma cold chain shipping needs. Csafe deliveries use active and passive bulk air cargo, parcel, cell and gene and specialty last-minute use cases that can meet the complete range of pharma cold-chain shipping requirements with quality and reliability.
Additionally, Csafe operates from over fifty service centers worldwide. The company has built a reputation as a proven partner for biopharmaceutical manufacturers looking to simplify supply chain woes. This includes a track record for finding shipping solutions for six billion doses of a COVID-19 vaccine.
“We’re excited to work with a global partner with a strong history of reliability and performance and a deep dedication to innovative therapies,” said BioLife Solutions CEO Mike Rice. “We have the best LN2 technology and cGMP storage facilities in the market, in addition to our other world-class CGT solutions, and we are confident our collaboration with CSafe will extend our reach and result in even more reliability and real-time service for CGT partners.”
The cold chain CGT market was worth USD$1.3 billion in 2021. The market is anticipated to reach USD$2.8 billion at a compound annual growth rate of 14.29 per cent until 2027.
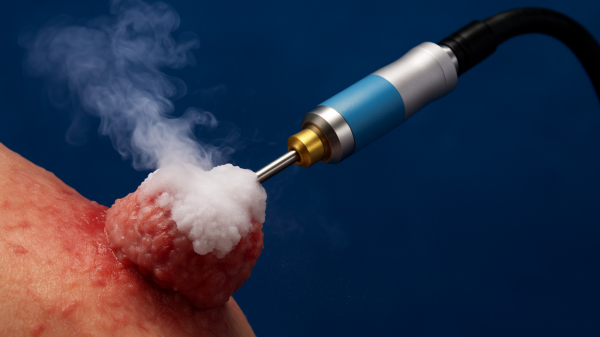
Medical tech curiosity IceCure gets regulatory nod to operate in Brazil
IceCure Medical (NASDAQ:ICCM) is a company with a curious bit of technology. It uses something called cryoablation technology, which it has called the ProSense System. It destroys tumours by freezing as an alternative to surgical removal.
IceCure announced that its ProSense cryoprobes and introducers have received regulatory approval from Agência Nacional de Vigilância Sanitária (ANVISA), the Brazilian health regulatory agency, to operate in said country. The company originally submitted the application in June 2022 through KTRFIOS IMPORTACAO E EXPORTACAO LTDA, IceCure’s distributor in Brazil
“We are very pleased to achieve this first step in the path to receiving full regulatory approval in Brazil. Working with KTRFIOS has been highly productive, and we anticipate strong demand in Brazil upon the additional pending approval of the ProSense Cryoablation System,”said Eyal Shamir, IceCure’s CEO.
The ProSense cryoprobes and introducers are disposal Class II devices as per ANVISA. Doctors insert cryoprobes into the body to freeze tumours, after which, introducers go to work on the tumour tissue. However, the system is considered a Class III medical device and the application is presently waiting for approval.
IceCure’s distribution agreement with its Brazilian partner includes the guarantee of at least USD$6.6 million sales in five years.
Previously, the company sought regulatory approval to operate in Vietnam. It submitted a filing for its ProSense System and accessories with the Department of Medical Equipment and Construction (DMEC) of Vietnam’s Ministry of Health.
The application included benign and malignant tumours in the breasts, lungs, liver, kidneys, and musculoskeletal tumours.