The popular psychedelic lysergic acid diethylamide (LSD) has shown promise in treating major depressive disorder (MDD) in the latest clinical study led by Mind Medicine Inc (NASDAQ: MNMD) (NEO: MMED).
On Friday, the company presented data in Basel, Switzerland showcasing the positive results from its Phase II LSD study for the treatment of the mental health condition.
MindMed says the primary data revealed substantial, swift, long-lasting, and favourable impacts of LSD on those involved.
The clinical trial examined the safety and effectiveness of the psychoactive drug in 61 patients. Patients were given 100 micrograms on their initial dosing day and 200 micrograms on their second dosing day after a four-week interval. Researchers then saw statistically and clinically significant improvements in their symptoms.
Read more: Numinus Wellness YoY revenue increased five times to $5.4M
Read more: Dutch government establishes MDMA research commission
MindMed says the positive results derived from this study have direct relevance to the company’s MM-120 LSD D-tartrate program for the treatment of generalized anxiety disorder (GAD).
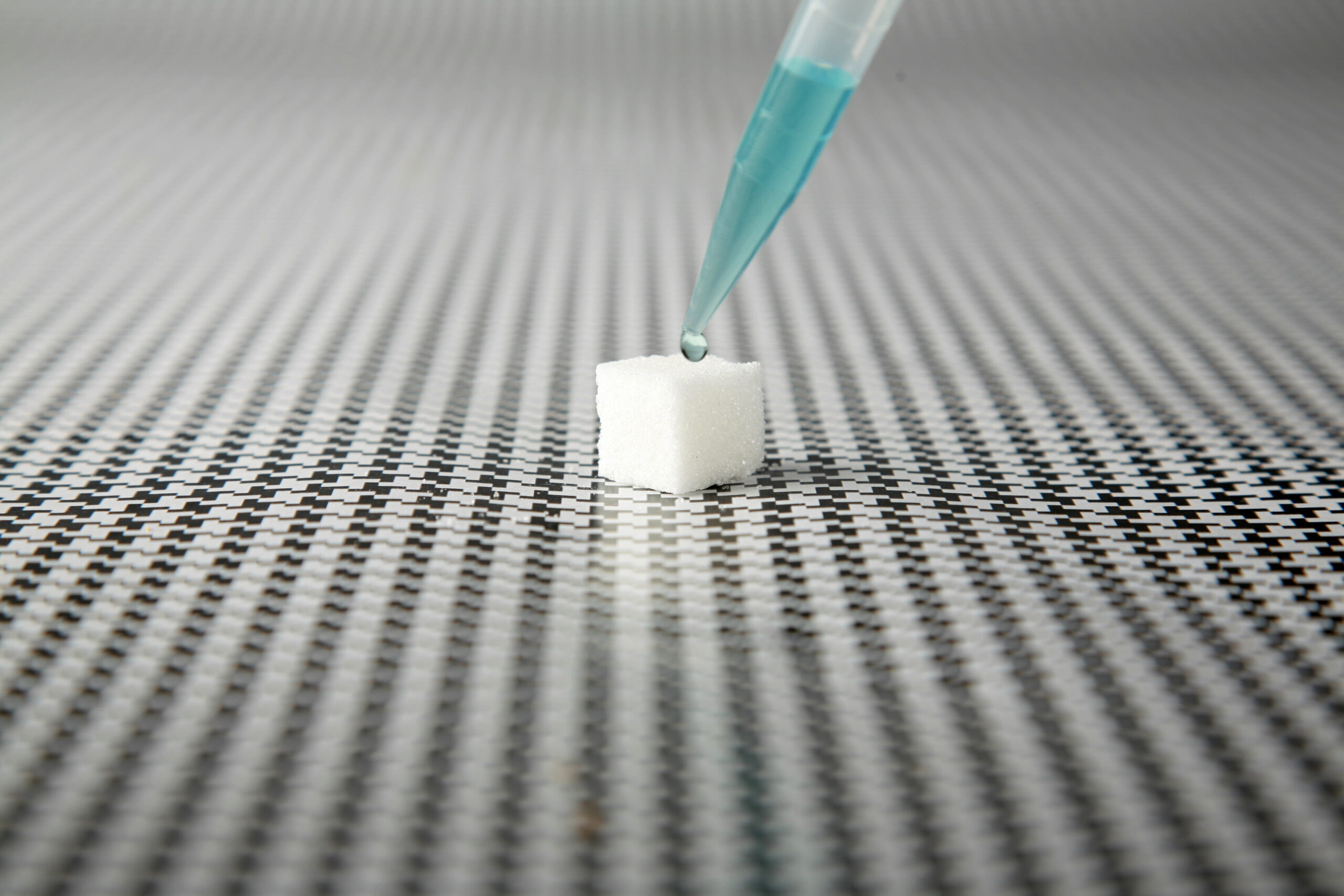
LSD tartrate is found in the common variety of “blotter” LSD or acid used on paper squares and sugar cubes, according to data from the United States National Library of Medicine provided by the University Hospital Basel (UHB) in Switzerland where the trial was held.
“The statistically and clinically significant improvements observed in this study reinforce preliminary findings that have shown the clinical potential of lysergide (LSD) in anxiety, depression and other brain health disorders,” said MindMed’s CEO and Director Robert Barrow.
“These positive findings are particularly relevant to our MM-120 program in generalized anxiety disorder, given the high degree of comorbidity of GAD and MDD.”
MindMed dosed its first patient with MM-120 in August last year in a study with 200 participants.
“We are extremely encouraged by the results we presented today, which demonstrate the strong, rapid and enduring improvements of this compound in patients suffering from MDD. We look forward to publishing the completed results in a peer-reviewed journal along with additional analyses,” said Matthias Liechti, Co-Primary Investigator of the trial at UHB.
Liechti has been actively involved with studying LSD for several years now.
Prof. Dr. Matthias Liechti | University Hospital Basel
VIDEO: https://t.co/HpJ6Xdu63X
Current Research on LSD in Healthy Subjects and Patients
Members access to all of the conference videos at once in the members area – https://t.co/fAfYfnEXzz#LSD #psilocybin #MINDfoundation pic.twitter.com/Qa2mydGCiI— MIND Foundation (@mind_europe) October 12, 2019
Read more: Psilocybin access denied to patients despite ongoing lawsuit against Health Canada
Read more: MindMed re-establishes Nasdaq listing requirements
MindMed re-established its Nasdaq listing requirements last September following a 1-for-15 reverse share split of its stock in August and a subsequent drop in the company’s share price afterward resulting in it falling below the minimum US$1.00 requirement on the exchange.
A 2022 study published in the journal Neuropsychopharmacology by researchers from UHB found that synthetic psilocybin was very similar to LSD, with the only significant difference being that LSD takes effect more rapidly and lasts longer.
MindMed’s stock took a sharp decline Friday on the OTC Markets, dropping by 62.12 per cent to US$0.025.
However, the company’s stock rose by 1.94 per cent to US$3.16 on the Nasdaq Stock Exchange.
rowan@mugglehead.com