Inspira Technologies (NASDAQ: IINN) (NASDAQ: IINNW) started the manufacturing process for its ALICE CPB (Cardiopulmonary Bypass) device with the endgame being its entry into the U.S. Food and Drug Administration (FDA) for 510(k) clearance.
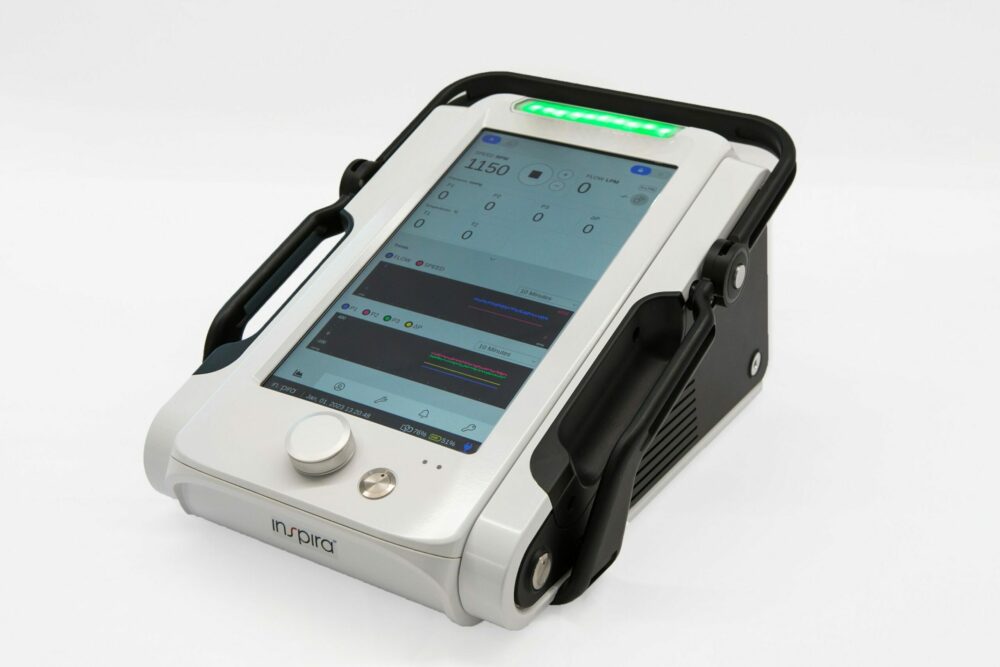
The company built the ALICE for clinical settings requiring CPB support. It incorporates several of Inspira’s features and capabilities into one device. One such capability is the HYLA Blood Sensor. The HYLA Blood Sensor performs continuous blood measurements in real time, potentially minimizing the need to collect blood samples from patients and assisting physicians monitor a patient’s clinical condition.
Conversely, a 510(k) is a premarket submission made to the FDA. Accordingly, the submitter demonstrates its prospective product is safe and effective. Also, submitting companies compare its device to other similar legally marketed devices and make and support equivalent claims.
The submitter can’t take their device to market until it receives the order declaring a device substantially equivalent (SE). The SE determination usually takes 90 days and is made based on the information submitted.
It intends the ALICE to be the first device to use the HYLA Blood Sensor to perform non-invasive, continuous measurements and alert physicians if there’s any serious changes.
If the FDA gives clearance, the device’s production line will expand to include low-rate initial production. Specifically, the operational stage supports serial manufacturing, quality control and shipping. Also, Inspira intends to assemble additional units for deployments in the U.S. and Israel.
“Today, we believe that we have achieved a very important milestone in line with our strategy towards the production and delivery of Inspira Technologies’ products to the market,” said Avi Shabtai, Inspira Technologies’ chief operating officer.
Read more: The Mugglehead technology roundup: medical tech and biotechnology edition
Read more: Inspire Medical Systems recruits top tier investor relations talent
Inspira bumps production quantity in time for FDA assessment
In this situation, Inspira reached out to a third-party offering New Product Introduction (NPI) services for medical electronic device companies. Accordingly, these services include full turn-key manufacturing, full service integration, including printed circuitboard manufacturing, assembly services, testing and packaging in facilities that meet Good Manufacturing Practices (GMP) compliance standards.
The ALICE device also provides advantages in terms of medical device design. A few examples include a large touchscreen and colorful graphics interface that helps increase visibility and functionality of data. The lightweight, highly durable device possesses a long-lived battery.
Inspira Technologies’ overall goal is to set a new standard for various aspects of patient care. Specifically, its tech targets intensive care units, general medical units, operating theatres, emergency medical services and small urban and rural hospitals. The focus is to boost accessibility to medical care for millions of patients worldwide.
The company is actively working to establish partnerships with globally ranked health centres to find endorsement and clinical adoption for regional uses for its CPB product, and blood measuring products and technologies.