Canada’s latest report on compliance and enforcement of cannabis laws — the first full annual report under the Cannabis Act — shows increased inspections of personal medical licensees than before legalization.
On Wednesday, Health Canada released the summary of inspections under the Cannabis Act, covering the timeframe of April 1, 2019 to March 31, 2020.
During that period, 417 inspections were carried out — 295 of those were rated either compliant or non-compliant, based on the number and severity of observations noted.
Inspections can include visually examining a facility, inventory, equipment, packaging, labelling and websites, as well as collecting and reviewing documents and records and taking samples for laboratory analysis
Most inspections (165) fell under the category of regular, which are conducted to monitor and ensure compliance overall. Target inspections, which focus on particular areas under the Cannabis Act, were the second-most reported (108).

Inspections during 2019–2020. Chart via Health Canada
Licence holders are expected to take corrective actions for noted issues of non-compliance during or after inspections. If not, they may be given a warning letter, lose their licence or have their products seized.
According to the latest summary, Health Canada took the following measures:
- issued five warning letters of non-compliance to licence holders requiring corrective measures,
- conducted 20 seizures and detentions of products,
- suspended three licences, and
- revoked two licences due to a non-compliance rating.
Read more: Nearly $8M of cannabis, Health Canada licences seized in Ontario raid
Inspections spike for personal medical licensees
The number of regular and targeted inspections has risen since Health Canada’s previous summary, but one category has seen significantly more inspections since the 2018–2019 report.
In the following year, inspections of licensees for personal and designated production of cannabis for medical purposes jumped over 800 per cent, from nine to 82.
Overall, the 2018–2019 report documented 293 inspections, about 40 per cent less than the new report.
The 82 inspections on personal medical licensees were conducted in Quebec (44), Ontario (22), British Columbia (14) and Manitoba (2).
From those, 91 observations were made:
- 21 inspections resulted in no observations, and
- 61 inspections resulted in 1 to 3 observations for each inspection.
The previous report covers a transition period of regulations and when full legalization came into play in Canada.
Prior to the Cannabis Act coming into effect in October 2018, Health Canada was guided by the Access to Cannabis for Medical Purposes Regulations (ACMPR), as well as the Food and Drugs Act and Controlled Drugs and Substances Act (CDSA).
April 1, 2018 to Oct. 16, 2018:
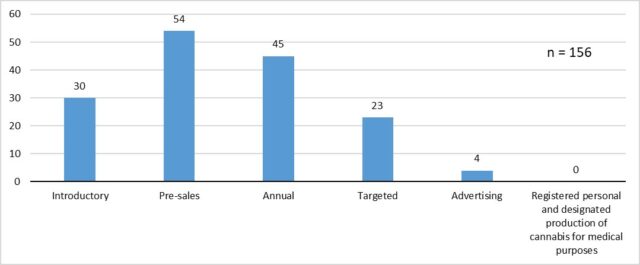
Number of inspections conducted by inspection type under CDSA and ACMPR from April 1, 2018 to Oct. 16, 2018. Chart via Health Canada
Oct. 17, 2018 to March 31, 2019

Number of inspections conducted by inspection type under the Cannabis Act and its Regulations
from Oct. 17, 2018 to March 31, 2019. Chart via Health Canada
In an email to Mugglehead, Health Canada notes that more inspectors were hired and trained, allowing for more inspections.
“Health Canada takes a risk-based approach to our regulatory oversight which can sometimes lead to fluctuations in our overall annual inspection targets. These changes are expected as this is part of inspection planning in any given year,” continues the email.
In the report, Health Canada has included steps it’s taking to “strengthen the oversight of persons authorized to produce a limited amount of cannabis for medical purposes.”
One of the steps is enhancing verifications of applications when there are risk factors to public health and safety, including when a high amount is authorized and applications with production sites that aren’t in a primary residence.
Along with sharing of information between health authorities and collaborating with “key stakeholders,” such as law enforcement and municipalities, Health Canada’s approach includes “applying new powers to refuse or revoke a registration on the grounds of public health and public safety increasing the focus on compliance promotion with registrants.”
Read more: Health Canada hints at increased grey market enforcement
Health Canada’s follow-up to inspections includes sending compliance letters to educate licence holders on the requirements under the cannabis laws.
Also in the latest report, there were 57 sales inspections, and five promotions inspections.
The regulator adds that it conducted 234 “promotions-related compliance verification activities,” which resulted in 130 actions taken, including four warning letters, 18 compliance emails and 108 compliance promotion emails or calls.
Health Canada says it proactively engages with regulated parties on the topic of compliance. Over the reporting period, the regulator says it completed 60 compliance promotion calls, seven compliance promotion letters and 22 compliance promotion sessions.
Update (2022-2-4, 11:45 a.m.): This article has been updated to include response from Health Canada.
Follow Kathryn Tindale on Twitter
kathryn@mugglehead.com














