South Korea has made its mark in the lung cancer breath diagnostics field with a machine that has demonstrated a 95 per cent level of accuracy.
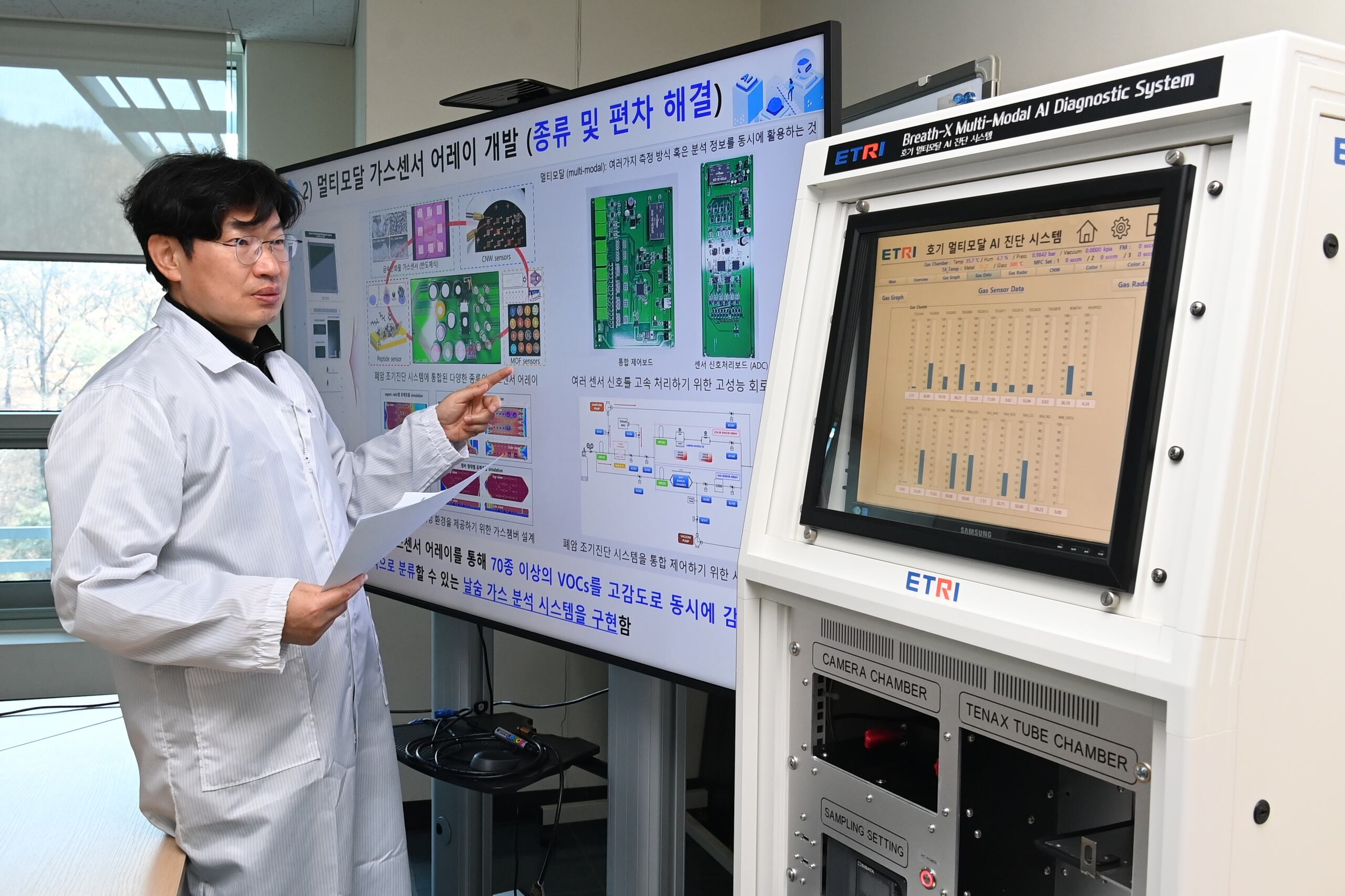
The nation’s government-funded Electronics and Telecommunications Research Institute (ETRI) has developed an artificial intelligence-powered apparatus for early stage lung cancer detection. They have named it the Breath-X Multi-Modal AI Diagnostics System.
This machine uses 70 sensors to detect volatile organic compounds (VOCs) associated with the disease in a breath sample. The process is completed within only 20 minutes using a deep learning algorithm.
The ETRI has been working on its development for a decade in partnership with Seoul National University’s Bundang Hospital.
Investigators at the institute are currently planning to complete additional clinical studies with over 1,000 participants for further validation. They say it has the potential to serve as a diagnostics tool for other types of cancer too.
“It is cheaper and faster to produce compared to existing hospital diagnostic equipment,” the research team at ETRI said.
Read more: Breath Diagnostics onboards new president and closes critical financing
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Breath testing has multiple advantages
This method of lung cancer diagnosis is particularly advantageous because it is non-invasive and radiation-free.
As highlighted by the South Korean researchers, breath testing equipment is significantly cheaper to manufacture than low-dose computed tomography (LD-CT) machines, without high maintenance costs.
One of the institute’s high-ranking officials pointed out that it could be economically beneficial once it becomes commercialized.
“It is expected to help reduce the government’s health insurance expenditure costs,” senior ETRI researcher, Lee Dae-sik, highlighted.
ETRI is not the only Asian organization experimenting with this type of diagnostics. A group of scientists from Zhejiang University in neighbouring China have been having success with a novel breath test of their own recently.
Early detection offered by these testing modalities is especially beneficial when considering the low survival rate lung cancer patients have. Just under 19 per cent will live for five years after receiving their diagnosis.
Read more: Scientists develop breath-related diagnostic tool for early detection for silicosis
Read more: Korean consortium propels lung cancer drug development with AI, supercomputers
North American companies pioneer others
One particular company in Kentucky has developed a breath analysis device that can deliver highly sensitive results in half the time that it takes the South Korean machine. It yields them in only 10 minutes.
The OneBreath system from Breath Diagnostics and its patented microreactor technology is capable of converting VOCs into more easily detectable forms. This helps to substantially improve sensitivity.
“OneBreath has the potential to complement or even replace traditional lung cancer screening methods such as LD-CT and PET scans,” Breath Diagnostics CEO, Ivan Lo, recently explained, “integrating AI-driven image evaluation to enhance diagnostic accuracy.”
At the Memorial Sloan Kettering Cancer Center (MSKCC) in the eastern United States, researchers have created a breath examination device called the E-Nose. Like the others, it is inexpensive to manufacture, and one test only costs about US$10.
“We anticipate this non-invasive, low-cost, portable, and highly reliable diagnostic method will revolutionize the diagnosis and clinical management of early-stage lung cancer,” MSKCC thoracic surgeon, Gaetano Rocco, said in June last year, “making it accessible to more patients, especially those resistant to CT scans or for whom a biopsy is infeasible.”
Memorial Sloan is currently trying to pool funding together for further research and development.
Additionally, Breathe Biomedical, based in New Brunswick, has been developing a breath analysis system as well. It prioritizes breast cancer detection, but it has the potential to be tailored for lung cancer applications.
rowan@mugglehead.com