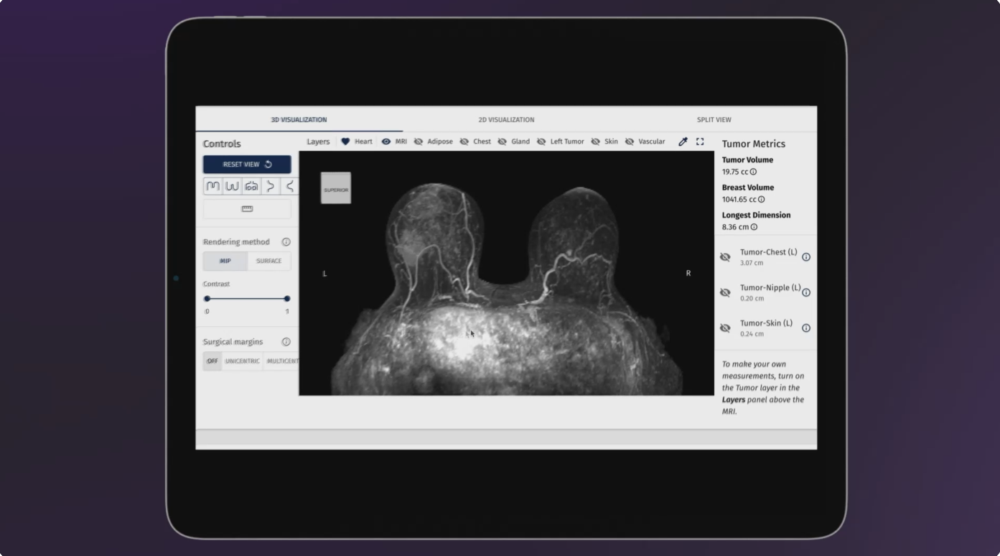
A clinical artificial intelligence company received the FDA nod for a technology that will improve surgical outcomes through careful conversion of standard breast MRI into 3D visualizations.
Announced on Wednesday, SimBioSys received a third FDA 510(k) clearance for its TumorSight Viz platform, which will help surgeons make more informed decisions regarding care.
TumorSight Viz 1.3 builds on the platform’s FDA-cleared foundation by adding new capabilities that enhance performance and usability. It significantly improves accuracy, making it more effective in complex surgical procedures.
Additionally, it expands clinical utility across a wider range of tumor types. The update also simplifies integration into existing surgical workflows, reducing setup time and training requirements. These improvements position TumorSight Viz 1.3 as a more versatile and efficient tool for surgical teams.
TumorSight Viz 1.3 represents the next evolution of a clinically validated platform designed to improve breast cancer surgery. Furthermore, the update expands functionality to support wider integration across surgical settings. It also builds on strong clinical validation to ensure reliable performance in real world use.
“As the leading cancer diagnosed in women worldwide, breast cancer presents a complex and urgent challenge demanding tools that enhance surgical clarity and enable more individualized care,” said Stacey Stevens, President and CEO of SimBioSys.
Stevens continued in saying that TumorSight Viz 1.3 gives surgeons a new level of intelligence and insight, allowing them to operate with greater confidence and tailor care to each patient’s unique anatomy and goals.
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Read more: Breath Diagnostics takes aim at lung cancer with One Breath
Platform processes cases in minutes
TumorSight Viz 1.3 sets a new standard with superior AI driven segmentation that delivers industry leading accuracy in lesion identification and tumor volume estimation. This precision enables surgeons to plan procedures with greater confidence.
In addition, the platform processes cases in minutes, offering same day insights that support timely, informed conversations with patients. New PACS connectivity further streamlines workflow by automating image transfers and reducing manual tasks, which boosts efficiency for both physicians and staff.
Furthermore, a growing body of clinical evidence supports TumorSight Viz. Studies show strong concordance with radiologist annotations, accurate tumor size delineation relative to breast volume, and consistent performance across more than 1,600 retrospective cases from over nine institutions.
“This latest version brings a new level of clarity and control to breast cancer surgery—delivering the anatomical insight and intuitive design surgeons have long needed,” said Barry Rosen, MD, FACS, breast surgical oncologist.
“It empowers us to make more informed decisions about margins, incisions, and reconstruction, and just as importantly, it helps patients visualize their care in a way that fosters trust, understanding, and confidence in the path forward.”
Rising expectations around precision and cosmetic outcomes are reshaping breast cancer surgery.
Re-excision rates remain above 20 per cent, highlighting the need for more accurate planning. At the 2025 ASBrS Annual Meeting, data showed wide variability in surgical decisions emphasizing the need for standardization.
Additionally, the National Comprehensive Cancer Network now recommends evidence based strategies for reconstruction and oncoplastic procedures. TumorSight Viz supports this shift by transforming complex MRI data into clear, anatomy specific insights. As a result, surgeons can operate with greater consistency, accuracy, and personalization, helping improve outcomes and reduce unnecessary procedures.
Read more: Breath Diagnostics opens Respiratory Innovation Summit with captivating presentation
Read more: Breath Diagnostics now offering a compelling investment opportunity
Breath Diagnostics targets $3M capital raise
Several companies are producing technologies similar to TumorSight Viz, focusing on using artificial intelligence to help fight cancer.
One notable example is Breath Diagnostics Inc, a U.S. based medical technology company developing OneBreath, a non invasive breath analysis platform. OneBreath uses volatile organic compound (VOC) profiling to detect early stage lung cancer. The aim here is to improve diagnostic accuracy and reduce the need for invasive biopsy procedures. Furthermore, the company targets high risk populations and seeks to integrate its solution into routine clinical workflows.
Breath Diagnostics Inc. introduced a Regulation CF crowdfunding campaign in May 2025, targeting up to USD$3 million by offering common shares at USD$3 each, with a $20,001 minimum raise. Proceeds will fund R&D, clinical trials, regulatory steps, and commercial setup.
Another key player is Ibex Medical Analytics, which uses artificial intelligence to enhance pathology workflows. Its Galen platform supports the detection of prostate, breast, and gastric cancers by analyzing pathology slides and finding malignancies with high accuracy. Ibex has joined with hospitals worldwide to streamline diagnoses and reduce human error.
PathAI uses machine learning to improve pathology diagnostics, particularly in breast and colorectal cancers. Its AI powered platform assists pathologists in identifying cancerous cells with greater consistency and speed.
Furthermore, Imagion Biosystems (ASX: IBX) is developing nanoparticle based imaging technologies to detect cancers like breast, ovarian, and prostate. Its MagSense technology aims to enable earlier and more precise tumor localization, potentially improving surgical outcomes.
These companies represent a growing field of data driven innovation transforming cancer care from diagnosis to treatment.
.
joseph@mugglehead.com