IceCure Medical (NASDAQ:ICCM) submitted a regulatory filing with the Department of Medical Equipment and Construction (DMEC) of Vietnam’s Ministry of Health for the ProSense System and accessories.
The application’s reach includes both benign and malignant tumours in the breasts, lungs, liver, as well as kidney cancer, ablation of cancerous or malignant tissue, musculoskeletal tumours and other indications. Liver, lung and breast cancer were the three most prevalent cancers in Vietnam in 2020, according to the World Health Organization.
“Asia is a significant market for ProSense and Vietnam is one of the fastest growing healthcare markets in the region. Having started the regulatory process in Vietnam, we anticipate high interest from potential distributors in the country,” said Eyal Shamir, IceCure CEO. “This comes on the heels of our recent successes in the region with Shanghai Medtronic Zhikang Medical Devices Co. Ltd. in China and Terumo in Japan, Singapore, and Thailand.”
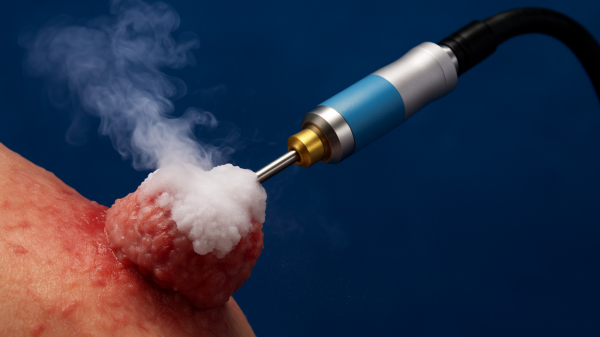
IceCure Medical builds, develops and markets ProSense, a cryoablation therapy that uses liquid nitrogen to treat both benign and malignant tumours by freezing. The primary focus areas include the breasts, kidneys, bone and lung cancer. It’s a minimally invasive technology that’s also a safe and effective alternative to hospital surgical tumour removal, and easily performed in a relatively short procedure.
According to the Mayo Clinic, cryoablation involves the insertion of a thin, wand-like needle called a cryoprobe, through the skin and into the tumour. After which, a gas is pumped through the probe to freeze the issue, which is then allowed to thaw. The freezing and thawing process is then repeated multiple times during the same treatment session.
Cryoablation is typically used when surgery isn’t an option, and can be used as a primary treatment for:
- Bone cancer
- Cervical cancer
- Eye cancer
- Kidney cancer
- Liver cancer
- Lung cancer
- Prostate cancer
At present, the ProSense system is marketed and sold worldwide for the above named indications, each of which has been cleared by the U.S. Food and Drug Administration (FDA) as well as in Europe.
This is a respectable move for the company as Vietnam’s medical device market was valued at USD$1.4 billion in 2019, and is expected to grow 10 per cent annually through 2024 with overall healthcare expenditures in the country totalling $17 billion, according to the U.S. Department of Commerce.