The best way for medical professionals to access psilocybin for training purposes is to sign up for a clinical trial, according to federal regulator Health Canada.
But advocates and experts say that’s unethical, and a waste of resources for everyone involved.
The authority tells Mugglehead that medical professionals should no longer seek exemptions under section 56 of the Controlled Drugs and Substances Act (CDSA), which up until recently had been the only route to legally access illicit substances for training and therapy.
Instead, Health Canada says healthcare workers should apply to a clinical trial it recently approved.
Despite not showing on its public-facing database, the agency has approved a clinical trial for synthetic psilocybin that healthcare workers can sign up for.
“Clinical trials not only protect the best interests of participants by ensuring that the controlled substance complies with [Good Manufacturing Practices] and is administered in accordance with national and international ethical, medical and scientific standards, but it can also build a body of research evidence on the safety and efficacy of unapproved drugs,” an agency spokesperson explains.
On Jan. 7, ATMA Journey Centers Inc. said it had been granted a no objection letter regarding its first sponsored trial, looking into the safe use of synthetic psilocybin in healthy, licensed healthcare practitioners enrolled in psychedelic-assisted therapy programs.
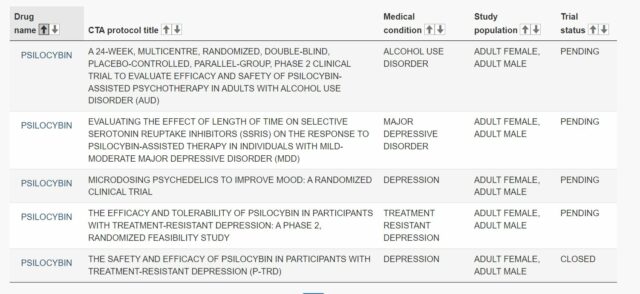
Currently listed clinical trials for psilocybin. Screenshot via Health Canada
Directing medical professionals looking to train with psilocybin to clinical trials is unethical, says UBC research board
In a letter sent to federal Health Minister Jean-Yves Duclos on Tuesday, psychedelics firm Numinus Wellness Inc. (TSX: NUMI) (OTC: NUMIF) urgently requested a mechanism, outside clinical trials, to allow therapy trainees to experience psilocybin as part of their training.
Numinus says it’s received independent advice from a member of the University of British Columbia’s research ethics board, “who confirmed that it was not ethical to conduct a clinical trial for therapist training in the absence of a specific research question,” reads the letter signed by Numinus CEO Payton Nyquvest, chief medical officer Evan Wood and UBC Okanagan VP of Indigenous initiatives and reconciliation Lindsay Farrell.
The ethics board says it’s routinely confronted with the question of fair use of time and resources when there is no clear benefit of a trial to study participants or society at large.
Read more: Vancouver Island University to launch Canada’s first psychedelic-assisted therapy graduate program
Read more: Canadian advocates denounce ‘flawed’ system for legal psilocybin access
“There is also the additional context of the responsibility of Health Canada to establish pathways for access that recognize the sovereign rights of Indigenous Peoples under the United Nations Declaration on the Rights of Indigenous Peoples (UNDRIP) in their historical and ongoing use of certain sacred medicines here on Turtle Island,” reads the letter.
“For obvious reasons, it is not ethical to restrict access to psilocybin experiential training for any self-identified Indigenous practitioners to clinical trials.”
"Invalid research is unethical because it is a waste of resources…"
Are we really going to conduct research to determine if psychotherapeutic training helps psychotherapy?
Bravo @NuminusWellness. Thank you for supporting Therapists and patients. @jyduclos https://t.co/nCLZxYXf5t
— SpencerHawkswell (@SpencerHawkswe1) February 15, 2022
Advocates will continue to push for other routes outside clinical trials
Victoria-based non-profit TheraPsil, which helps patients and medical professionals access medical psilocybin, has raised concerns about how some patients with life-threatening conditions, or medical professionals, aren’t approved under any stream of access, nor have time to wait for a clinical trial to open up.
“While our team fully supports clinical trials, we also realize that our Canadian health care system is not set up in a way that makes clinical trials accessible for a majority of Canadian healthcare professionals and patients,” TheraPsil said in a statement on Tuesday.
Read more: ATMA to run clinical trial for synthetic psilocybin on medical professionals
Read more: Health Canada says patients should access medical psilocybin via clinical trials
On Jan. 31, the Health Canada sent a letter stating it will refuse access to psilocybin for 86 healthcare professionals who are enrolled in a TheraPsil training program. The advocacy group says some of them had waited almost a year for a response.
Health Canada says the letter was issued as a result of the recently authorized clinical trial.
TheraPsil says it will continue to advocate for urgent responses for patients and healthcare professionals who don’t have the time to wait for approval or the option to join a trial.

A number of licensed producers, like Numinus Wellness can now provide psilocybin to patients through the Special Access Program. Photo via Numinus
Access to psychedelics determined on a case-by-case basis
On Jan. 5, Health Canada enacted amendments to its Special Access Programme (SAP), which allows patients suffering from a serious or life-threatening condition to access illicit drugs such as psychedelics for therapy when other treatments have failed or are unavailable.
In the past, patients, medical professionals and advocates have expressed confusion over the steps the agency takes to determine who gets access to psilocybin.
“In the case of the Special Access Program, clinical pharmacists and licensed physicians with a variety of medical backgrounds are involved in the evaluation of requests,” a Health Canada spokesperson explains, referring to a recently published guidance document.
The agency says it authorizes or denies requests on a case-by-case basis, using the information provided by the physician who helps the patient apply. The application has to include sufficient evidence supporting safe use, the nature of the medical emergency, the availability of marketed alternatives as well as how the information provided supports the request for a specific condition.
Read more: Canada’s first set of approved health workers begin psilocybin therapy training
Read more: What amendments to Canada’s restricted drug program mean for patients and practitioners
Follow Natalia Buendia Calvillo on Twitter
natalia@mugglehead.com













