Kentucky-based medtech startup Breath Diagnostics Inc is raising USD$3 million through regulation crowdfunding, reaching out to the public to get them involved in the fight against cancer.
Regulation crowdfunding is one of the avenues that a startup or private company can invite the public, including non-accredited investors, to help raise funds for a product or cause. Typically, these are smaller amounts but it gets an investor some skin in the game with a company involved in an issue they care about. Like Breath Diagnostics.
“Through Regulation CF, we invite you to invest in Breath Diagnostics and help bring this life-saving technology to market,” said the company.
“This opportunity isn’t limited to accredited investors—anyone can join our mission. Your support will bring us closer to a future where we catch lung cancer early and treat it successfully.”
Why Breath Diagnostics? Because over the past 30 years, cancer death rates have steadily declined. That’s good news.
However, this trend is reversing in a post-COVID world. Cancer is increasingly affecting younger people, not just older patients.
Doctors call cancers diagnosed in people under 50 “early onset” and global incidence has surged by 79 per cent since 1990. This is a problem. Especially since doctors haven’t yet identified the exact cause of this rise in early onset cancer.
The one pertinent critical takeaway moving forward is that early detection improves patient outcomes and lowers total healthcare costs.
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Read more: Breath Diagnostics takes aim at lung cancer with One Breath
Younger patients often miss out on cancer screenings
Most people don’t realize that the Centers for Disease Control and Centers for Medicare & Medicaid Services report that lab tests make up only three per cent of healthcare spending. Yet these tests influence about 70 per cent of medical decisions.
Younger patients often fall outside the recommended age for routine cancer screenings. As a result, they face more advanced and aggressive stages of cancer by the time they’re diagnosed.
Lung cancer stands out as increasingly common, deadly, and expensive to manage.
Common assumptions are that it’s a smoker’s disease and if you quit smoking, you’re free and clear. Except researchers estimate that 10 to 20 per cent of U.S. lung cancer cases occur in people who never smoked. In some Asian countries, that number reaches as high as 95 per cent.
The statistics are staggering.
According to the American Cancer Society, lung cancer kills over 130,000 people each year in the United States. That’s more people than breast, prostate, and colorectal cancers combined.
Globally, the numbers scale. Lung cancer is the leading cause of cancer-related deaths worldwide. In 2022, it was responsible for an estimated 1.8 million deaths globally, accounting for approximately 19 per cent of all cancer deaths.
However, if doctors catch lung cancer early, the five-year survival rate reaches 65 per cent. If it’s late, survival drops to as low as five per cent. Yet, despite clear benefits of early screening, over 50 per cent of high-risk smokers never receive a screening recommendation.
Even among those who do, only about six per cent actually get screened.
Why? Lots of reasons.
Read more: Breath Diagnostics onboards new president and closes critical financing
Read more: Focus on diet for lung cancer prevention isn’t strong enough, research shows
Anxiety over radiation exposure isn’t overblown
Participants who opted out of lung cancer screening cited five main barriers.
Transportation issues and the distance to screening facilities were often the primary reason for cancellations, as patients couldn’t get a ride. Also concerns over radiation exposure and the possibility of false-positives, which could lead to invasive procedures.
Typically, the anxiety over radiation exposure isn’t overblown either. Recent studies have raised concerns about the safety of low-dose CT scans, the current standard for lung cancer screening. The radiation exposure compared to traditional CT scans may be greatly reduced, but it isn’t zero. Therefore, this can still pose long-term risks.
Experts worry that repeated exposure, especially in high-risk populations, could increase the likelihood of developing cancer in the future.
Furthermore, some research suggests that in some cases the potential harm from the radiation may outweigh the benefits. This has prompted calls for alternative screening methods that minimize radiation while maintaining diagnostic accuracy.
And that’s before we get into the prevalence of false positives.
For example, it can take up to 50 CT scans to find one case of lung cancer.
CT scans often detect small lung nodules and more than 95 per cent of these nodules turn out to be non-cancerous. These nodules are typically the results of past infections, smoking or other past conditions.
Radiologists must then investigate each finding to rule out cancer. That’s expensive, often painful and exposes patients to more radiation. It also leads to unnecessary, invasive procedures for benign nodules. These risks and costs often outweigh the benefit of CT scans for lung cancer screening in many cases.
As such, safer screening technologies are definitely in high demand.
Breath Diagnostics and its OneBreath technology represents a significant alternative.
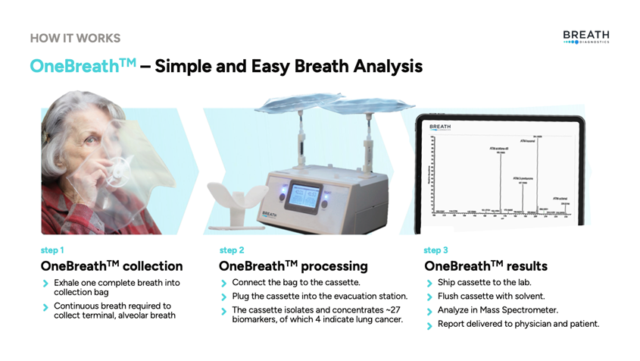
Image via Breath Diagnostics.
OneBreath is non-invasive and radiation free
OneBreath is a patented, breath-analysis platform that rapidly detects certain diseases without radiation or invasive procedures. The proprietary microreactor accurately identifies lung cancers—outperforming traditional CT scans and PET imaging by minimizing false positives and improving early detection.
How does it work?
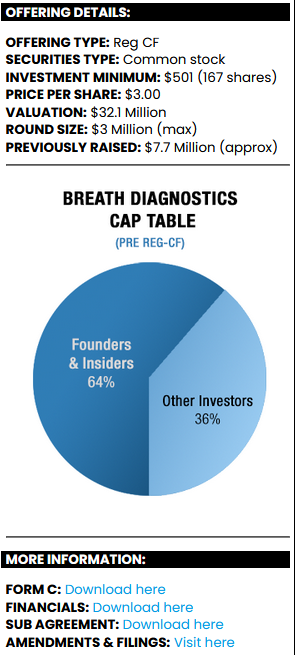
Image from Breath Diagnostics.
It involves one exhaled breath into a bag. The results are processed by the microreactor. After which, the device chemically stabilizes the volatile organic compounds (VOCs) in the breath.
The VOC’s are then analyzed using ultra-high-performance liquid chromatography-mass spectrometry. This allows for precise identification of biomarkers associated with early-stage lung cancer. The process delivers results with 94 per cent sensitivity and 85 per cent specificity, and a reduction of false positives compared to conventional CT scans.
Additionally, the company has taken steps to validate the technology through extensive trials involving over 800 patients. These trials have demonstrated that it outperforms traditional methods like CT scans in identifying malignant tumours.
Now the company is reaching out to both traditional investors and non-investors alike for the next stage.
The upsides are substantial
For example, the technology could disrupt the USD$150 billion global cancer diagnostics market by addressing significant unmet needs, especially in lung cancer screening. It also has many other uses. The technology can potentially detect other diseases, including pneumonia, tuberculosis, and additional cancers, which may increase future revenue streams.
Management estimates it will take about three years to complete FDA approval and fully commercialize the pneumonia indication.
They expect lung cancer applications, including screening and indeterminate pulmonary nodules, to take an additional three to four years.
Since its inception Breath Diagnostics has raised USD$7.7 million with helpful grants from the Bill and Melinda Gates Foundation and others, and is sitting at a market cap of USD$32.1 million.
It’s early days yet and there’s a long way to grow.
Visit https://invest.equifund.com/offering/breathdiagnostics/details to learn more.
.
joseph@mugglehead.com