The psychedelic manufacturer Silo Pharma, Inc. (Nasdaq: SILO) has started an exploratory feasibility study to evaluate the intranasal patented drug SPC-15 that works in serotonin receptors and is meant to reduce stress.
The company announced the study for the drug on Tuesday, which acts on the serotonin type 4 (5-HT4) receptor. Silo aims to have the Investigational New Drug (IND) submission to the Food and Drug Administration on target for the first quarter of next year.
The receptor plays an important role in regulating mood, anxiety and cognition. Drugs that activate this receptor have fast-acting antidepressant (AD)-like effects in preclinical models, according to a study by Nature. Drugs that act on it are known to be agents that act on serotonin receptors in the intestine and promote intestinal movement and emptying.
Silo’s SPC-15 was developed in collaboration with Columbia University and is meant to be used against stress and uses “biomarkers” for the treatment of stress-induced affective disorders, anxiety and PTSD.
Recent research in psychedelics such as psilocybin and LSD has shown that the substances act on the cortical 5-HT2A receptors.
Read more: Silo Pharma to develop ketamine implant for fibromyalgia and chronic pain
Read more: Silo Pharma prepares pre-IND package for FDA on novel ketamine formulation
“We believe our research partner’s patented proprietary nasal-to-brain technology could be well suited for the delivery of SPC-15, and we expect to receive the results of these in vitro lab tests by the end of September,” Silo Pharma CEO Eric Weisblum said.
“If the results are positive, we will work to prepare an Investigational New Drug (IND) application for submission to the FDA in the first quarter of 2024.”
Silo Pharma focuses on developing psychedelic medicines and research for people suffering from Post Traumatic Stress Disorder, Alzheimer’s disease and other rare neurological disorders.
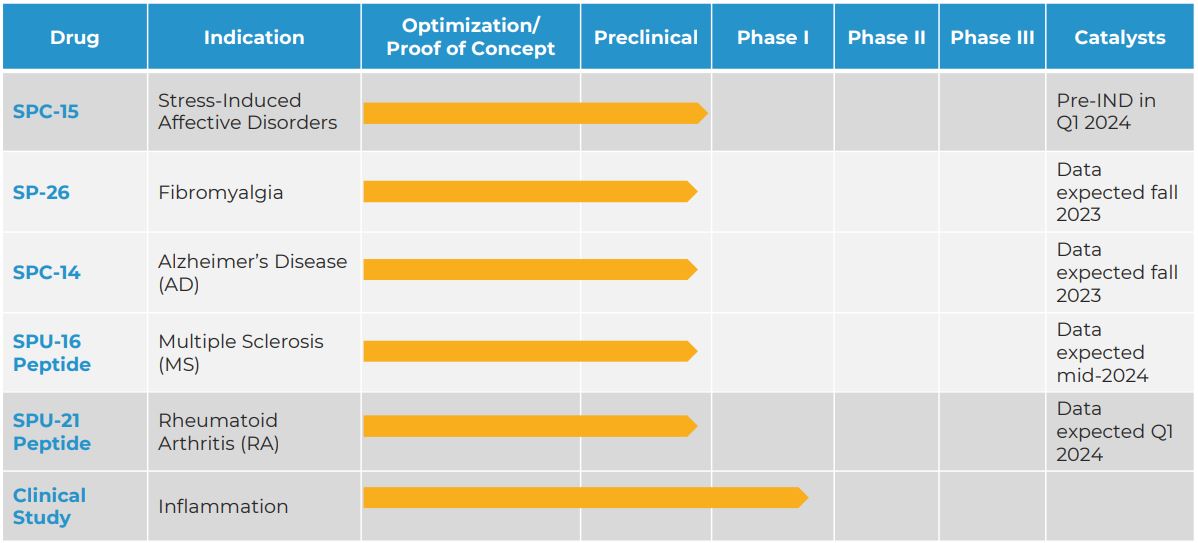
Current drugs that are being developed by the company. Table via Silo Pharma.














