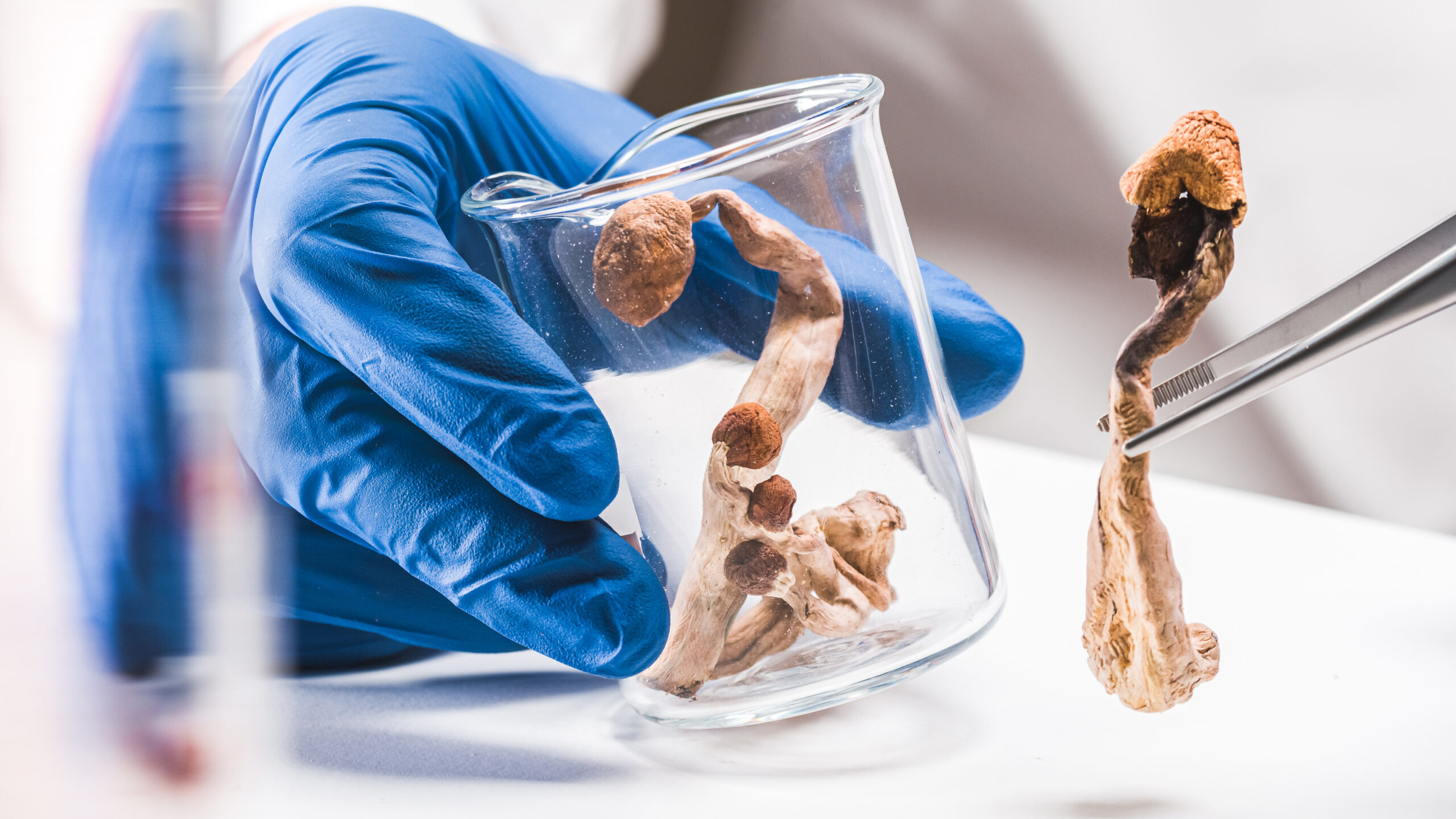
United Kingdom-based MGC Pharmaceuticals Ltd (ASX: MXC) (LSE: MXC) has obtained permission from the Slovenian Ministry of Health to engage in research with psilocybin and says it is the first company operating in Slovenia to do so.
MGC announced the development on Monday and plans to develop new drug formulations with the psychedelic compound at its Slovenian research and development facility.
The company says it will now be able to bring the psychedelics industry one step closer to the pharmaceutical industry in Slovenia through the development of new psilocybin-based medicines. The company’s Chief Commercial Officer Robert Clements spoke about the news in an interview with Proactive Investors.
$MXC MGC Pharmaceuticals' focus "will not change" despite psilocybin news https://t.co/2YAb7yVLPN @mgc_pharma $MGCLF #MXC #MGCLF
— Proactive (@proactive_UK) April 4, 2023
Read more: Dutch government establishes MDMA research commission
Read more: Psilocybin access denied to patients despite ongoing lawsuit against Health Canada
“We are pleased to have obtained permission from the Slovenian Ministry of Health to undertake pharmaceutical research with psilocybin, which puts the company at the forefront of experimental, pharmaceutical research,” said Roby Zomer, CEO and Managing Director of the company.
“We are extremely grateful to the Slovenian health ministry for enabling us to utilize our research expertise in this area,” he added.
MGC has a significant patient base in Australia where psilocybin will be a prescribable treatment for certain mental health conditions as of July this year.
MGC’s stock rose by 10 per cent on Wednesday to AUD$0.011 on the Australian Securities Exchange.
Europe and the U.K. have been key contributors to the psychedelics landscape in recent days
Last month, the federal government in the Netherlands established a research commission to study MDMA and its potential therapeutic uses. The commission will be presenting the findings from its multidisciplinary assessment to the Dutch Cabinet by the end of January next year.
In February, the United Kingdom’s Awakn Life Sciences Corp. (NEO: AWKN) (OTCQB: AWKNF) (FSE: 954) signed an agreement with an anonymous party to help establish a series of psychedelics clinics in Portugal.
An anonymous client in the U.K. has received or will soon be receiving a large shipment of MDMA (300 grams) from Canada’s PharmAla Biotech Holdings Inc. (CSE: MDMA) for research purposes.
Read more: Awakn Life Sciences helps establish psychedelics clinics in Portugal
Read more: PharmAla Biotech gets Health Canada approval to export large MDMA shipment to the U.K.
In November 2022, Australia’s Enosis Therapeutics partnered with Germany’s Ovid Clinics to offer virtual reality-modulated psychedelic therapy in Berlin.
In June last year, the Psychedelic Access and Research European Alliance was formed, comprising 16 different member organizations dedicated to progressing psychedelics science and research. Founding members included the Osmond Foundation, the European Brain Council and the Association of European Cancer Leagues.
Later in October 2022, the European Psychiatric Association joined PAREA as well. The EPA is the foremost association representing psychiatry in Europe and has more than 78,000 members.
rowan@mugglehead.com