A young woman in Illinois sees the silver lining in her recent lung cancer diagnosis as an opportunity to educate others about the disease. Aurora Lucas was only 28 when symptoms first arose, which goes to show that it does not only impact people during their later years.
The phrase “anyone with lungs can get lung cancer” is certainly accurate. LUNGevity, a non-profit Lucas advocates with in her home city of Chicago, recently launched a campaign with this name to promote awareness about the importance of getting screened.
This initiative made its debut in November for Lung Cancer Awareness Month.
Thankfully for those who may find themselves in a similar position, new screening technologies that will become available in the near future will make prompt diagnosis a more convenient and easy process.
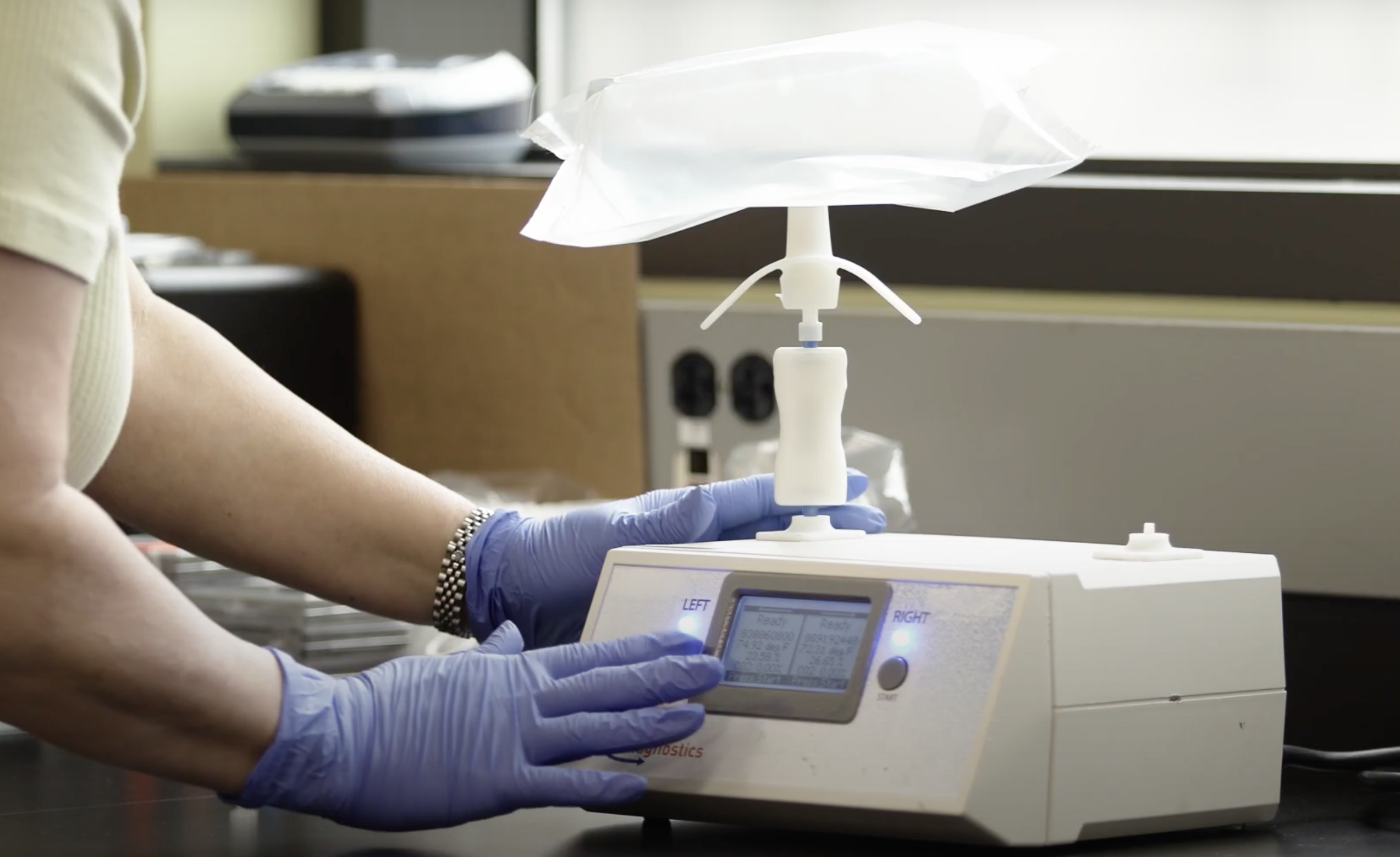
Breath Diagnostics stands out in this regard with its OneBreath system. This non-invasive, radiation and needle-free breath test could potentially render low-dose CT scans obsolete in the years to come once FDA approval has been attained and it becomes commercially available. Multiple clinical studies have validated the technology’s immense potential in the screening market.
For treatment in Aurora’s case, she underwent chemo, radiation and targeted oral therapies. This has enabled her to be alive and well today.
“Cutting-edge therapies have given me life, a second chance, and the ability to see a future,” Lucas stated in December.

Aurora Lucas is a renowned lung cancer awareness advocate. Photo credit: Lung Cancer Foundation of America
Read more: Breath Diagnostics onboards new president and closes critical financing
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Survival is continually becoming more likely
The American Lung Association has highlighted that the global lung cancer survival rate has risen by 26 per cent over the past five years. Screening innovation combined with innovative new treatments and surgical technologies are making this possible.
Lucas is a testament to this recent success.
But, the association has pointed out that only 27 per cent of cases are being detected in the early stages, highlighting the need for a greater number of awareness campaigns and detection tools.
In addition to breath-testing devices, spit analysis tools, AI-powered diagnostics systems and a variety of blood biopsies and urine tests have been helping to fill this void. Although low-dose CT scans are currently the only FDA-approved means of testing for the disease, new measures have been emerging that are less cumbersome.
rowan@mugglehead.com