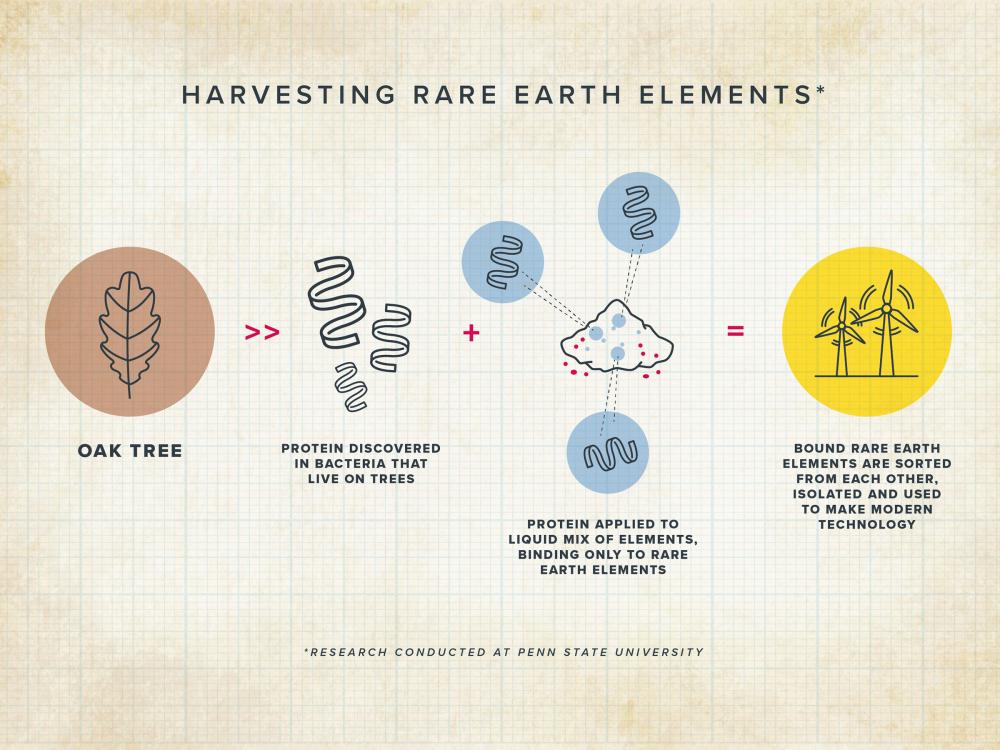
Rare earth elements like neodymium and dysprosium are popular in the mining industry because they are used in smartphones and hard drives but they are hard to separate from each other and the earth’s crust they were formed in.
However, researchers at Penn State University in Pennsylvania have found a mechanism using a bacterial protein that can separate rare earth elements faster and better than when done by humans or using toxic chemicals.
The protein is naturally occurring in the bacterium Hansschlegelia quercus which is isolated from English oak buds.
The research was led by Joseph Cotruvo Jr. who is an associate professor of chemistry at the university. The findings were published this month in the journal Nature.
“If you can harvest rare earths from devices that we already have, then we may not be so reliant on mining it in the first place,” Cotruvo said in a statement.
He explained that regardless of the source, the challenge of separating one rare earth from another to get a pure substance remains.
“Whether you are mining the metals from rock or from devices, you are still going to need to perform the separation. Our method, in theory, is applicable for any way in which rare earths are harvested,” he said.
Read more: Saskatchewan develops proprietary extraction cells for rare earth processing facility
Read more: New Tesla motor could disrupt rare earths sector
Unique reaction allows efficient separation
The culprit of the innovative method involves a protein which researchers are calling lanmodulin or LanM. When exposed to rare earth elements, it has a unique reaction that allows a more efficient separation and identification than previous methods.
The bacterial protein can bind to another unit of itself –a process called “dimerization”–when it is bound to certain rare earths, but prefers to remain a single unit, or “monomer,” when bound to others.
This mechanism can lead to greener, more sustainable mining processes that do not require toxic chemicals and will enhance recycling practices in the mining sector, researchers explained.
Though chemically alike, each rare earth element has unique applications in technology.
The standard process for separating rare earth elements into their pure forms requires hundreds of steps and uses hazardous chemicals like kerosene and phosphates, which are also found in pesticides and fire retardants. This complex and toxic separation method is necessary to isolate rare earth elements for industrial uses, even though they are closely related.
“There is getting them out of the rock, which is one part of the problem, but one for which many solutions exist,” Cotruvo said, adding that once the rare earth elements are out, another step is needed, the separation.
“This is the biggest and most interesting challenge, discriminating between the individual rare earths, because they are so alike.”
natalia@mugglehead.com