Olympus Corp of the Americas, a subsidiary of Olympus Corporation (TYO: 7733), has launched its most advanced single-use fine needle biopsy device in the United States, targeting ultrasound-guided tissue sampling.
The device aims to support diagnosis of difficult diseases, including pancreatic cancer, where precise sampling often determines treatment decisions.
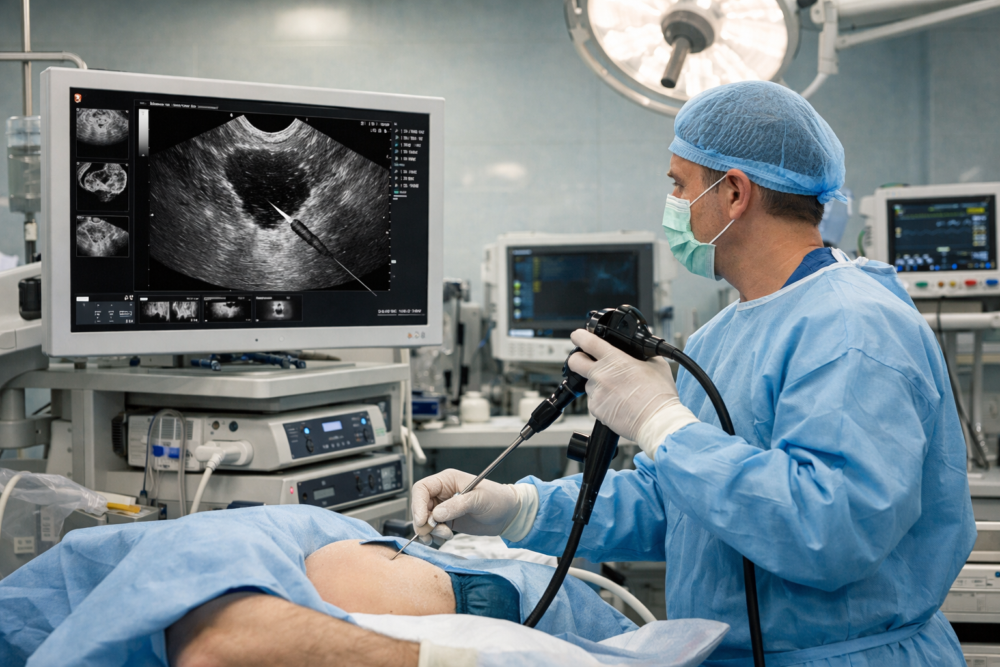
The launch introduces the SecureFlex fine needle biopsy needle, designed to collect larger, intact tissue samples from hard-to-reach anatomical areas.
Additionally, the needle supports access to challenging regions such as the pancreatic head and the uncinate process. Olympus offers the SecureFlex needle in 19-gauge, 22-gauge, and 25-gauge sizes to match different procedural needs. The company plans to present the device at two major U.S. medical events in early 2026.
Further, Olympus will showcase the needle at the Cedars-Sinai Endoscopy Symposium from Jan. 28 to 31. It will also demonstrate the device during Orlando Live Endoscopy 2026 from Feb. 4 to 6.
These events place the product in front of gastroenterologists who routinely perform complex biopsies.
The SecureFlex needle supports endoscopic ultrasound-guided fine needle biopsy, commonly known as EUS-FNB. EUS-FNB combines endoscopy and ultrasound to guide needles toward lesions that standard endoscopes cannot reach directly. Physicians typically insert the ultrasound scope orally and visualize tissue beneath the gastrointestinal wall.
Additionally, the method allows clinicians to collect tissue through the digestive tract wall without open surgery. This approach reduces recovery time while still providing samples suitable for detailed laboratory testing. The SecureFlex needle aims to improve the quality of those samples.
Read more: Breath Diagnostics pioneers novel lung cancer breath test
Read more: Breath Diagnostics leaders promote their mission at Miami investment conference
Targeted therapies and immunotherapies rely on detailed biopsy results
According to Olympus executives, gastroenterologists need dependable tools to maintain diagnostic accuracy in demanding cases. The company described the new needle as a solution designed to improve access and procedural efficiency. It said the design helps clinicians retrieve adequate samples, even in anatomically complex situations.
The SecureFlex needle features a Dual-Beveled Raptor Tip intended to cut tissue smoothly and precisely. The outer cutting edge supports controlled puncture, while the inner surface draws tissue into the needle. Consequently, the design helps preserve cellular structure compared with earlier needle models.
Additionally, the device uses a nitinol construction to resist bending during repeated passes. This material helps the needle maintain straightness within twisted or narrow anatomy. The needle also includes a multi-layer sheath designed to improve handling during procedures.
Further, houndstooth-patterned dimpling improves ultrasound visibility, helping physicians track needle placement in real time. These features aim to reduce uncertainty during delicate biopsy procedures. The device arrives as demand grows for higher-quality tissue sampling.
In recent years, targeted therapies and immunotherapies have increased reliance on detailed biopsy results. Accurate molecular analysis often requires larger, well-preserved tissue samples.
Consequently, biopsy tools now play a central role in personalized cancer treatment planning. The SecureFlex needle joins a broader gastrointestinal portfolio from Olympus Corporation.
That portfolio includes the EU-ME3 ultrasound processor, designed to deliver advanced imaging in a compact system. Additionally, the processor supports diagnostic imaging and ultrasound-guided interventions for hepatobiliary and pancreatic procedures.
Read more: Breath Diagnostics completes install of advanced mass spectrometry system
Read more: Prestigious medtech intelligence firm recognizes Breath Diagnostics for innovation
Non-invasive detection options on the rise
Beyond large needles, non-invasive cancer detection is rapidly evolving offering alternatives to painful tissue biopsies and inconvenient scans. These newer methods aim to catch disease earlier, reduce discomfort, and make screening more accessible for people at risk.
One promising approach analyzes breath instead of blood or tissue. Breath Diagnostics Inc. has developed OneBreath, a non-invasive system that can detect volatile organic compounds (VOCs) in a single exhaled breath. The technology uses a patented microreactor chemistry and ultra-sensitive analysis to reveal molecular markers linked to early-stage lung cancer. Clinical studies suggest the test delivers high sensitivity and specificity without needles, radiation, or long appointments,
Another company in this space, Owlstone Medical, focuses on breath analysis through its Breath Biopsy platform. Owlstone’s technology captures and analyzes VOC biomarkers in breath to uncover metabolic signals associated with disease. It is exploring breath tests for several cancers, including lung cancer, with research trials such as its EVOLUTION program identifying exogenous probes that could indicate tumour activity from breath samples.
Blood-based alternatives also grow in clinical use. Guardant Health (NYSE: GH) offers liquid biopsy tests that detect fragments of tumour DNA circulating in the bloodstream. These tests do not require surgical tissue extraction and can help identify genomic alterations linked to multiple cancer types. Liquid biopsies provide actionable molecular information and increasingly support treatment decisions in earlier stages of disease.
These non-invasive tools contrast sharply with traditional biopsies or imaging. Traditional tissue biopsies involve inserting needles or incisions to remove tissue for lab analysis, which can cause pain, carry complication risks, and require recovery time. Imaging scans like low-dose CT can detect abnormalities but may expose patients to radiation and still require follow-up biopsies for confirmation.
.