Multi-cancer screening operator Guardant Health Inc (NASDAQ: GH) (FRA: 5GH) has chosen to back the latest awareness campaign launched by the American Lung Association (ALA).
On Sept. 24, the ALA announced the debut of a lung cancer screening advocacy initiative aimed at educating people about the foremost problems related to diagnosing and treating the disease.
“Too many people are not receiving the comprehensive testing that can guide life saving treatments,” commented Lauren Lemmons, Vice President of Nationwide Corporate and Foundation Relations at the ALA.
The organization she works for highlighted that 23 per cent of lung cancer patients receive chemotherapy or radiation treatment before undergoing full biomarker testing. This is a significant issue than can result in less successful treatment and unnecessary complications, the ALA pointed out.
“Together we are raising awareness about lung cancer screening and biomarker testing and advancing our mission to defeat lung cancer,” Lemmons added.
Association President, Harold Wimmer, says the newly launched campaign was crafted based on feedback received from lung cancer patients and their families about why patients are not being screened with the latest diagnostic techniques and tools.
The ALA found that individuals with lung cancer were anxious due to a lack of available info, frustrated by communications with healthcare providers and did not understand the logic behind judgement about lung nodules suspected of being malignant.
“This new campaign responds directly to those concerns,” the association concluded in a news release announcement, “offering clear, evidence-based resources to people about lung cancer screening and biomarker testing.”
The reveal lacked specifics and was more focused on goals it would like to achieve. Guardant and ALA will be providing educational resources, the parties say.
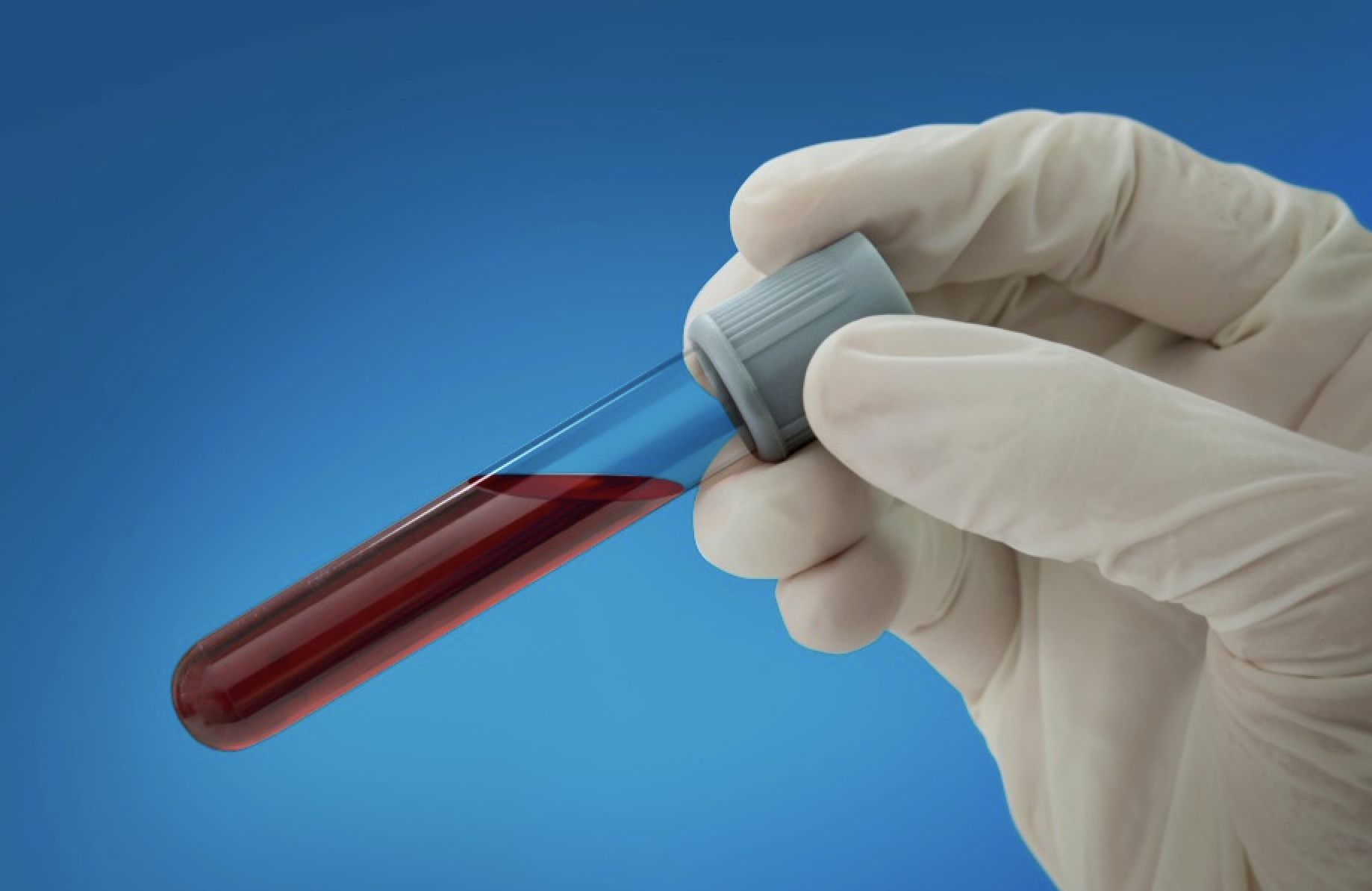
Nasdaq-listed Guardant has developed a blood biopsy that can detect circulating tumour DNA present in samples from individuals with lung cancer. Like other screening innovators involved with this medtech niche, such as Breath Diagnostics or bioAffinity Technologies Inc (NASDAQ: BIAF), the oncology operator aims to overcome issues associated with low-dose CT scans.
“At Guardant Health, we believe a simple blood test can improve lung cancer screening compliance by overcoming barriers associated with current methods such as low-dose CT scans,” the California-based company has specified, “while still being effective and more accessible.”
Guardant is currently in the midst of completing the “SHIELD [Screening for HIgh-frEquency maLignant Disease LUNG]” study. It is evaluating a lung cancer specific blood test. The investigation started in 2022 and is still ongoing.
Read more: Breath Diagnostics leaders promote their mission at Miami investment conference
Follow Rowan Dunne on LinkedIn
rowan@mugglehead.com