The University of Bath is set to begin a pioneering clinical trial in Edinburgh with serious potential downstream effects for how procedures like a lung biopsy are performed in the future.
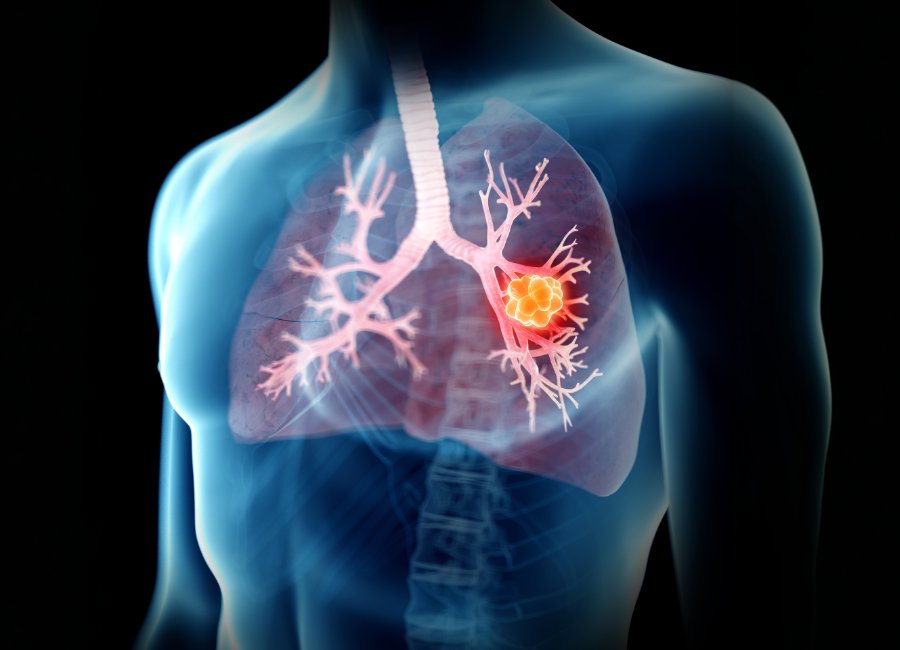
Announced on Tuesday, the study will evaluate a novel medical device designed to extract tissue from deep within the lungs of patients with suspected lung cancer, potentially transforming how the disease is diagnosed and treated.
Prothea Technologies, a medical technology spinout from the Universities of Bath and Edinburgh, leads the trial. The study will recruit 40 patients at the Royal Infirmary of Edinburgh to test an innovative endoscope incorporating a proprietary optical fibre developed at Bath. In addition, the endoscope is paired with advanced image processing equipment, allowing clinicians to examine lung tissue in real time and collect sufficient biopsies from suspicious areas.
A lung biopsy can confirm a diagnosis in the laboratory and reveal the molecular structure of a lesion. Consequently, doctors can select more targeted cancer treatments. Furthermore, the team plans to integrate a laser ablation catheter with the imaging device, potentially enabling clinicians to treat lesions immediately after diagnosis. This development could allow biopsies and treatment in a single visit, saving patients multiple hospital appointments.
“The first use of our technology in patients is an exciting milestone,” said Dr Jim Stone, chief technical officer and physicist at Bath.
“This is where we see how our device could improve tissue sampling for pathology.”
ACCORD co-sponsors the trial with the joint research office between the University of Edinburgh and NHS Lothian. Together, the project exemplifies collaboration between academic institutions and healthcare providers, advancing innovation in lung cancer diagnosis and treatment.
Read more: Breath Diagnostics tech achieves pneumonia prediction breakthrough in peer-reviewed study
Read more: Breath Diagnostics leader speaks at lung cancer education event in Louisville
Lung cancer diagnosis is often challenging
Lung cancer remains the leading cause of cancer related deaths worldwide, according to data from the World Health Organization (WHO). It accounts for approximately 18 per cent of all cancer deaths. In the US, it causes one in five cancer fatalities, surpassing the combined deaths from colon, breast, and prostate cancers. Despite advancements in treatment, survival rates remain low, making early detection critical.
However, diagnosing lung cancer is often challenging due to low compliance rates with screening protocols.
Studies show that adherence to annual screening declines over time. Unfortunately, annual adherence drops from 61 per cent in the first year to 51 per cent in the second year.
This decline reduces detection rates, particularly for early stage cancers. Factors contributing to low compliance include lack of awareness, perceived low risk, and logistical barriers. Addressing these issues is essential to improve early detection and enhance survival outcomes for lung cancer patients.
Several companies are developing technologies to overcome barriers in lung cancer diagnosis, particularly low compliance with traditional screening.
Owlstone Medical has created the Breath Biopsy platform, which analyzes volatile organic compounds in exhaled breath to detect lung cancer. In addition, their system can differentiate between cancerous and non cancerous conditions, offering clinicians a non-invasive tool for early detection. The company developed the platform for simplicity and speed, potentially increasing patient participation in screening programmes.
Meanwhile, Breath Diagnostics Inc. offers the OneBreath system, which captures and chemically transforms compounds in breath to deliver rapid diagnostics.
This non-invasive technology provides high sensitivity and specificity, enabling earlier detection of lung cancer than conventional methods. Furthermore, it reduces the need for hospital visits and invasive procedures, addressing logistical barriers that often hinder screening compliance.
.