IceCure Medical Ltd. (NASDAQ: ICCM) received a Notice of Allowance from the United States Patent and Trademark Office for a patent application called ‘Cryogen Flow Control.’
Once granted, the patent would be in effect until 2045. The company already has the patent for this invention in Japan and is waiting on further approval in the European Union and other markets.
IceCure’s CEO, Eyal Shamir, said the new invention aims to improve cryoablation efficiency and broaden future treatment uses. He noted the company is currently awaiting FDA approval to market ProSense for early-stage breast cancer. The targeted use involves women aged 70 and over when paired with adjuvant endocrine therapy.
Therefore, IceCure continues expanding its intellectual property portfolio to strengthen its cryoablation platform. The company also sees this as a key step in advancing its global commercial rollout. Shamir added that IceCure leads the world in liquid nitrogen-based cryoablation technology.
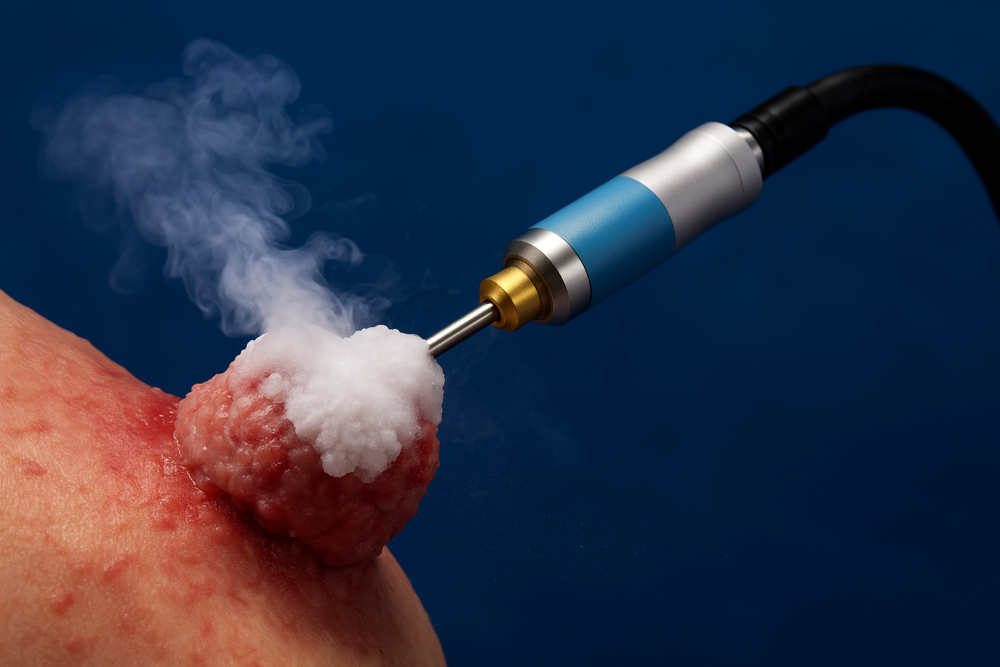
The ProSense Cryoablation System offers a minimally invasive way to destroy tumours through freezing. It uses liquid nitrogen to create large lethal zones, maximizing effectiveness against both benign and cancerous lesions in the breast, kidney, lung, and liver.
Precise temperature control plays a key role in ensuring both efficacy and tissue safety during cryoablation. This patent covers cryogenic flow control that uses sensor data to regulate cryogen flow. The system ensures the catheter or probe tip reaches and maintains the desired temperature.
This optimized delivery improves the effectiveness of cryoablation treatments. Additionally, advanced flow control systems can support navigation and mapping within the patient’s anatomy. Furthermore, developers can integrate these systems into a broad range of cryosurgical tools.
Read more: Breath Diagnostics opens Respiratory Innovation Summit with captivating presentation
Read more: Breath Diagnostics now offering a compelling investment opportunity
Over 300K new invasive breast cancer diagnoses in the US per year
The global market for cryoablation continues to grow rapidly, driven by rising cancer rates and demand for minimally invasive treatment options. In 2023, reports from marketing firms, Flair Insights and Verified Market Reports, both valued the global cryoablation market at approximately USD$2.4 billion. They project it will grow at a compound annual growth rate (CAGR) of 14 to 16 per cent. It could potentially reaching USD$6 to $7 billion by 2030.
ProSense offers a non-surgical solution that aligns well with these trends. It uses liquid nitrogen to freeze and destroy tumours in organs such as the breast, kidney, lung, and liver. Breast cancer alone represents a major opportunity.
Over 300,000 new cases of invasive breast cancer are diagnosed each year in the United States. Therefore, the need for alternatives to surgery—especially among older patients—is significant. Women aged 70 and above with early-stage breast cancer increasingly seek outpatient or office-based treatment options.
In addition, healthcare systems are shifting more procedures from hospitals to ambulatory surgical centers and office-based clinics.
This movement increases the appeal of ProSense, which offers a compact, portable, and efficient solution. Furthermore, cryoablation presents fewer complications than traditional surgery, especially for aging populations.
Also, improved reimbursement frameworks in the U.S. and other markets create additional tailwinds for commercial adoption. If the U.S. Food and Drug Administration grants approval for ProSense’s use in early-stage breast cancer, IceCure could see significant expansion in a growing global market.
.