One of the best ways to efficiently complete lung cancer surgery is to make undesirable disease cells glow with an intravenous drug, apparently. It becomes visible with infrared light during the procedure.
Surgeons from the OU Health Center at the University of Oklahoma announced that they had started utilizing Cyatlux this week. Philip Low, a researcher from Purdue University in Indiana, invented the oncology drug.
Cytalux was first used for ovarian cancer treatment before receiving FDA approval for lung cancer in 2022. It is manufactured by the company On Target Laboratories, which was founded by Low and has a license for use of his intellectual property.
“This technology allows us to visualize cancer that might otherwise be difficult to detect,” University of Oklahoma thoracic surgeon, Dr. J. Matthew Reinersman, said in a press release. “For lung cancer patients, it means we can perform less invasive surgeries with more precision, potentially leading to faster recoveries and better outcomes.”
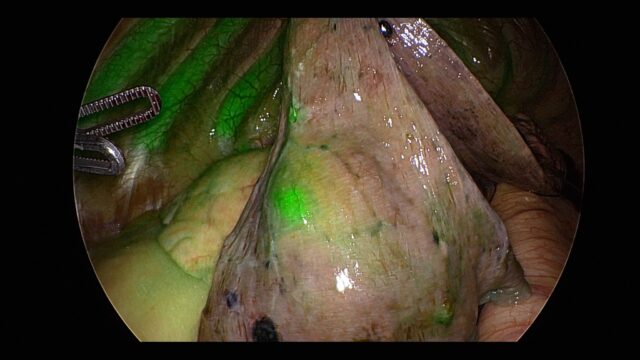
Lung cancer surgery photo. Credit: On Target Laboratories
OU Health says the drug enabled doctors to remove a lung nodule detected in a 69-year-old patient this September. The Cytalux-assisted surgery left him cancer-free and he did not need to receive chemotherapy or radiation treatment.
Surgeons at West Virginia University (WVU) just started using Cytalux to assist with the removal of lung cancer cells too. It enables them to remove as little healthy tissue as possible. They have administered the drug to over 50 patients.
Dr. Nicholas Baker, a thoracic surgeon from WVU, says he is now able to pinpoint about 8 per cent more cancerous lesions that other imaging technologies don’t detect.
Read more: Breath Diagnostics onboards new president and closes critical financing
Read more: Breath Diagnostics pioneers novel lung cancer breath test
American Lung Association honours Low with ‘Legacy Award’
The prominent health organization recognized him for his groundbreaking invention at its annual gala in Indianapolis this April. It is the only FDA-approved drug of its kind.
On Target Laboratories raised US$30 million in a Series C funding round at the beginning of 2024. The funding will help accelerate commercialization of the drug.
“This new targeted treatment is giving countless lung disease patients new hope,” Tanya Husain, Executive Director of the Indiana Lung Association, said in March.
Lung Cancer Awareness Month, ongoing for another 15 days, has helped shine the spotlight on the importance of getting screened for the disease and treating it swiftly.
rowan@mugglehead.com