Cannabis-based medicine Epidiolex could soon help even more Americans living with treatment-resistant epileptic disorders.
GW Pharmaceuticals plc (NASDAQ: GWPH) announced Monday it filed paperwork with the U.S. Food and Drug Administration to allow Epidiolex to be prescribed to treat seizures associated with Tuberous Sclerosis Complex.
Epidiolex has already been granted Orphan Drug Designation from the FDA for the treatment of focal and generalized seizures associated with TSC.
Read more: UK approves 2 cannabis-based medicines
Read more: GW Pharmaceuticals’ cannabis-based epilepsy drug gets EU approval
The FDA is expected to approve the application later this year, GW Pharmaceuticals CEO Justin Gover said in a statement.
“The submission … for Epidiolex is an important step towards the prospect of offering a new treatment option for those patients with TSC who battle difficult-to-treat seizures,” he added.
Epidiolex has been approved by the FDA to treat two other seizure-causing disorders, Lennox-Gastaut syndrome and Dravet syndrome.
Living with TSC
TSC is a rare genetic condition affecting around 50,000 Americans and one-to-two million people globally, according to the National Institute of Neurological Disorders and Stroke.
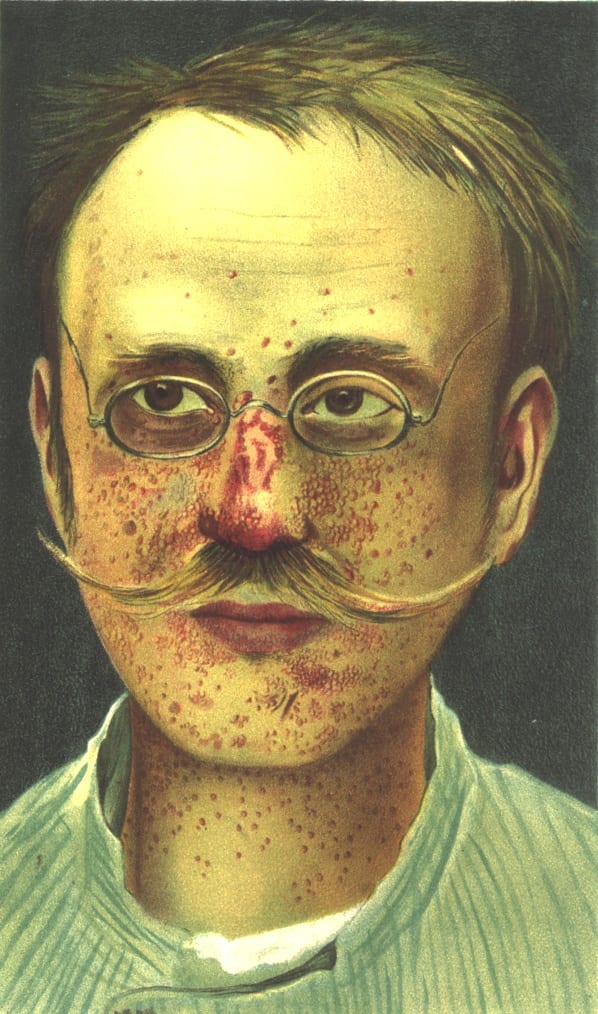
A patient with tuberous sclerosis in Munich 1903. Image via Wikimedia Commons.
It causes tumours to form on many different organs, mainly the brain, eyes, heart, kidneys, skin and lungs, Tuberous Sclerosis Alliance vice president of communications Jaye Isham told Mugglehead in an email.
It’s also the leading genetic cause of both autism and epilepsy but varies in severity between cases, even sometimes between identical twins, Isham said. Many people with TSC can work as doctors, researchers, lawyers and teachers, while others are severely effected, unable to live independently and require a lifetime of care, he added.
Up to 85 per cent of people living with TSC experience seizures, but more than 50 per cent of them do not respond to standard anti-epileptic medications and have uncontrollable epilepsy, Isham said.
“Therefore, additional treatments are always welcome,” he said, referring to Epidiolex.
At the American Epilepsy Society annual meeting GW Pharmaceuticals presented its study showing Epidiolex can reduce TSC-related seizures. In the study some patients were given a low dose, at 25 milligrams per kilogram they weighed, and a high dose, of 50 milligrams per kilogram each day. This translates to a daily dose of 1.75 grams per day, or 3.5 grams per day for the average adult, who weighs 70 kilograms.
The low dose reduced seizures 49 per cent, and the high dose 48 per cent, compared to the placebo group which saw around a 27 per cent reduction in seizures, according to a company press release.
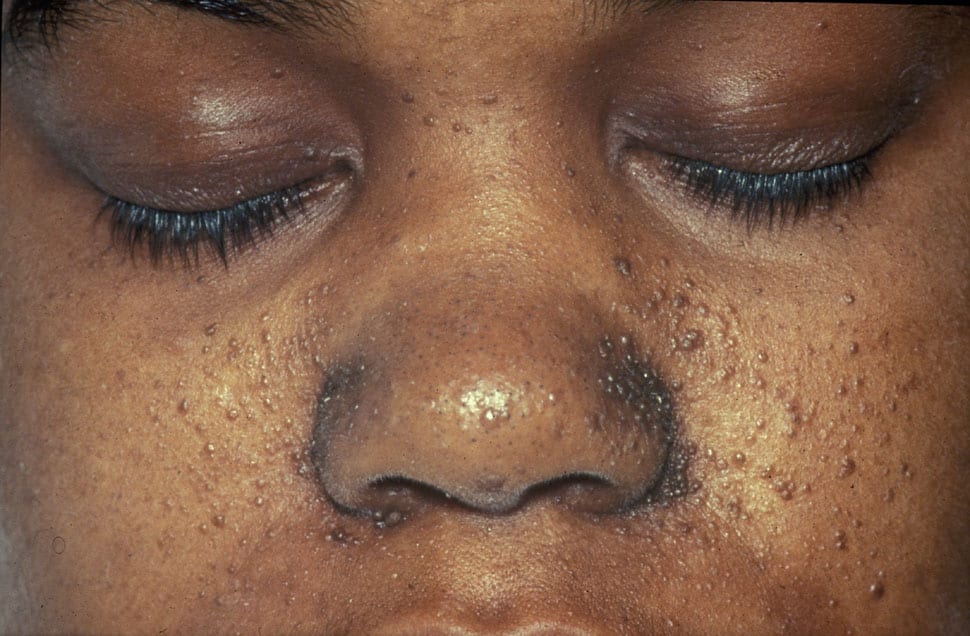
Cover photo: “A case of Tuberous Sclerosis showing facial angiofibromas in characteristic butterfly pattern,” via Wikimedia Commons.
michelle@mugglehead.com
@missmishelle